Answered step by step
Verified Expert Solution
Question
1 Approved Answer
14 aved ut of uestion In thermodynamics the Van der Waal s equation relates the mean pressure p of a substance to its molar volume
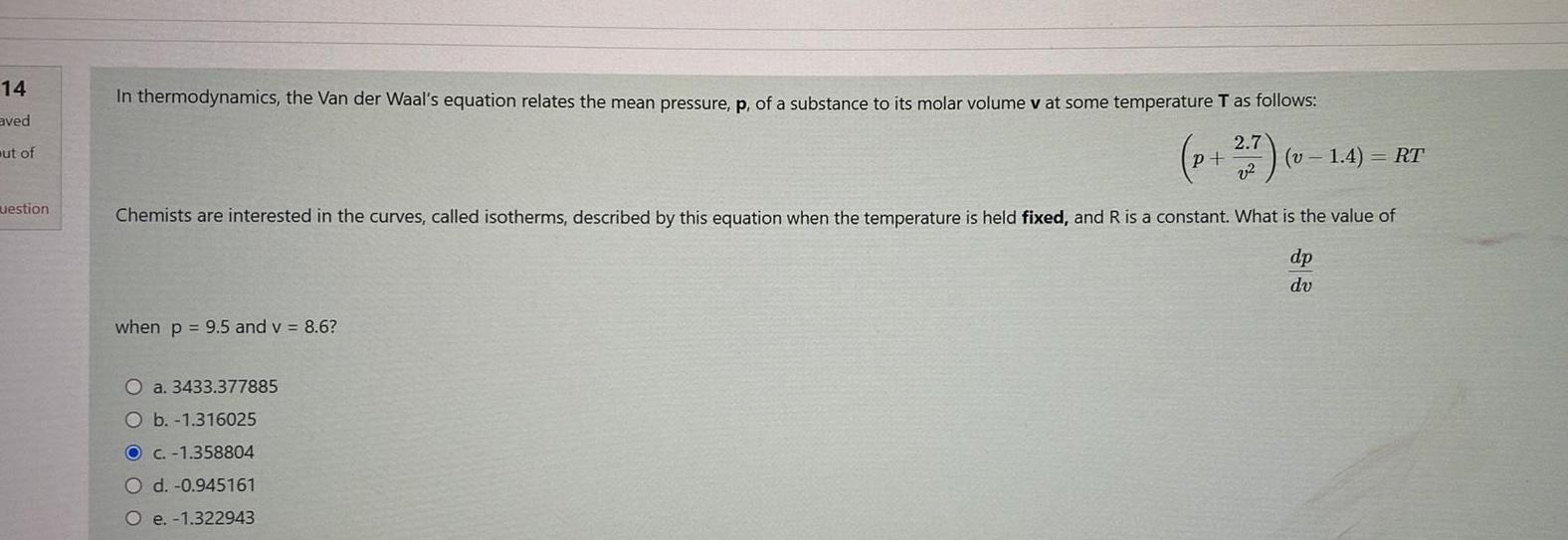
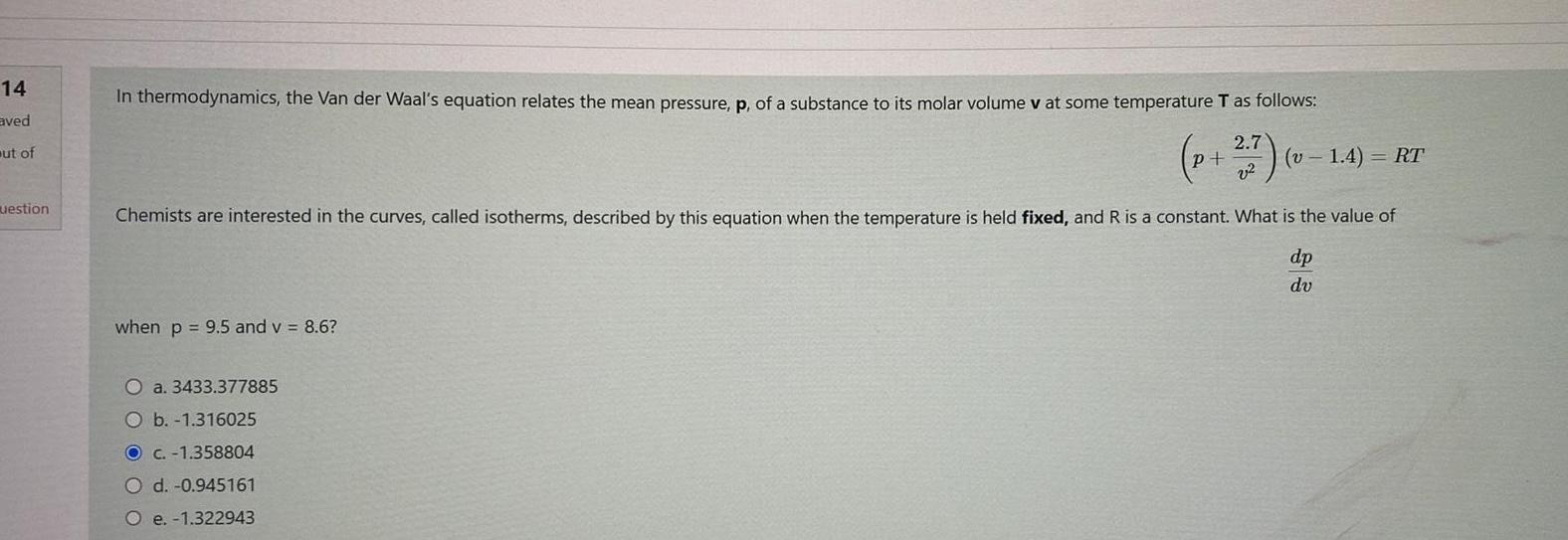
14 aved ut of uestion In thermodynamics the Van der Waal s equation relates the mean pressure p of a substance to its molar volume v at some temperature T as follows p 7 v v1 4 RT Chemists are interested in the curves called isotherms described by this equation when the temperature is held fixed and R is a constant What is the value of dp dv when p 9 5 and v 8 6 O a 3433 377885 O b 1 316025 O c 1 358804 O d 0 945161 O e 1 322943
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


