Question
(14%) Problem 4: Suppose you want to raise the temperature of a 0.185-kg piece of ice from -20.0C to 130C. The heat of fusion
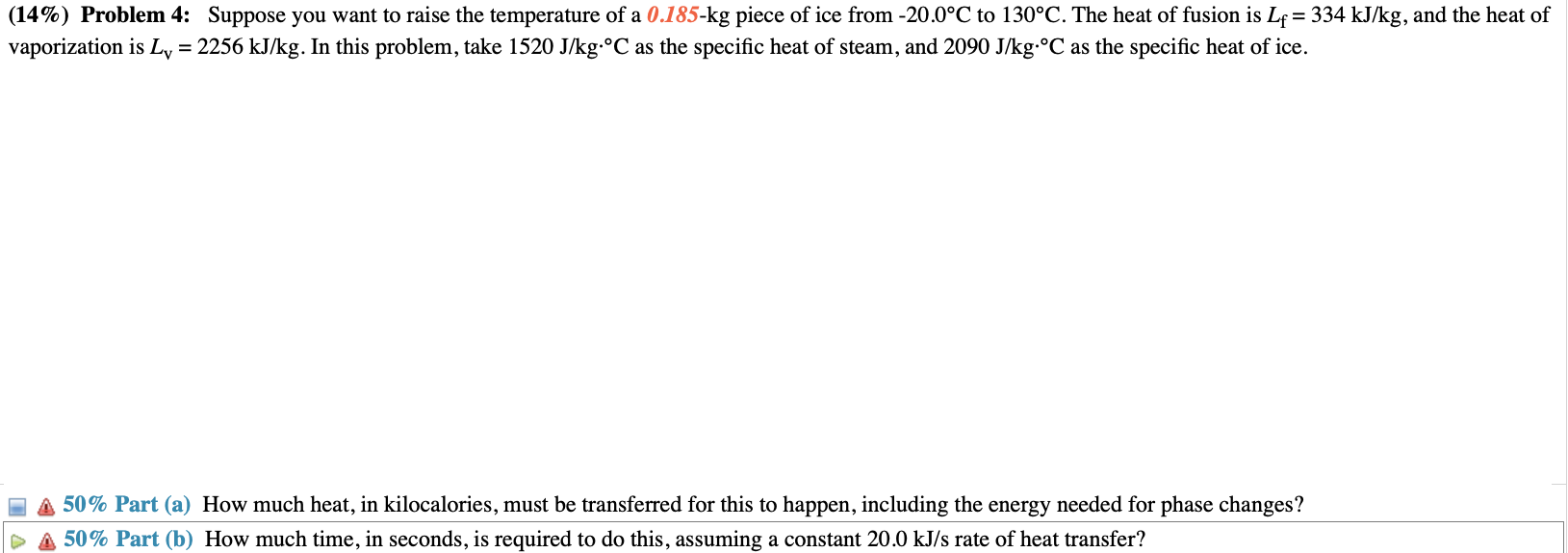
(14%) Problem 4: Suppose you want to raise the temperature of a 0.185-kg piece of ice from -20.0C to 130C. The heat of fusion is Lf = 334 kJ/kg, and the heat of vaporization is Ly = 2256 kJ/kg. In this problem, take 1520 J/kg C as the specific heat of steam, and 2090 J/kg C as the specific heat of ice. 50% Part (a) How much heat, in kilocalories, must be transferred for this to happen, including the energy needed for phase changes? 50% Part (b) How much time, in seconds, is required to do this, assuming a constant 20.0 kJ/s rate of heat transfer?
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Part a Heat Required Well calculate the heat needed for each stage of the process and add them toget...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Conceptual Physics
Authors: Paul G. Hewitt
12th edition
77652207, 0-07-811271-0, 9780077572150, 978-0077652203, 978-0-07-81127, 77572157, 978-0321909107
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



