Answered step by step
Verified Expert Solution
Question
1 Approved Answer
14 Question (1 Point) 5 What batch time is required to achieve 80% mole conversion for the following kinetics, if the beginning concentration of the
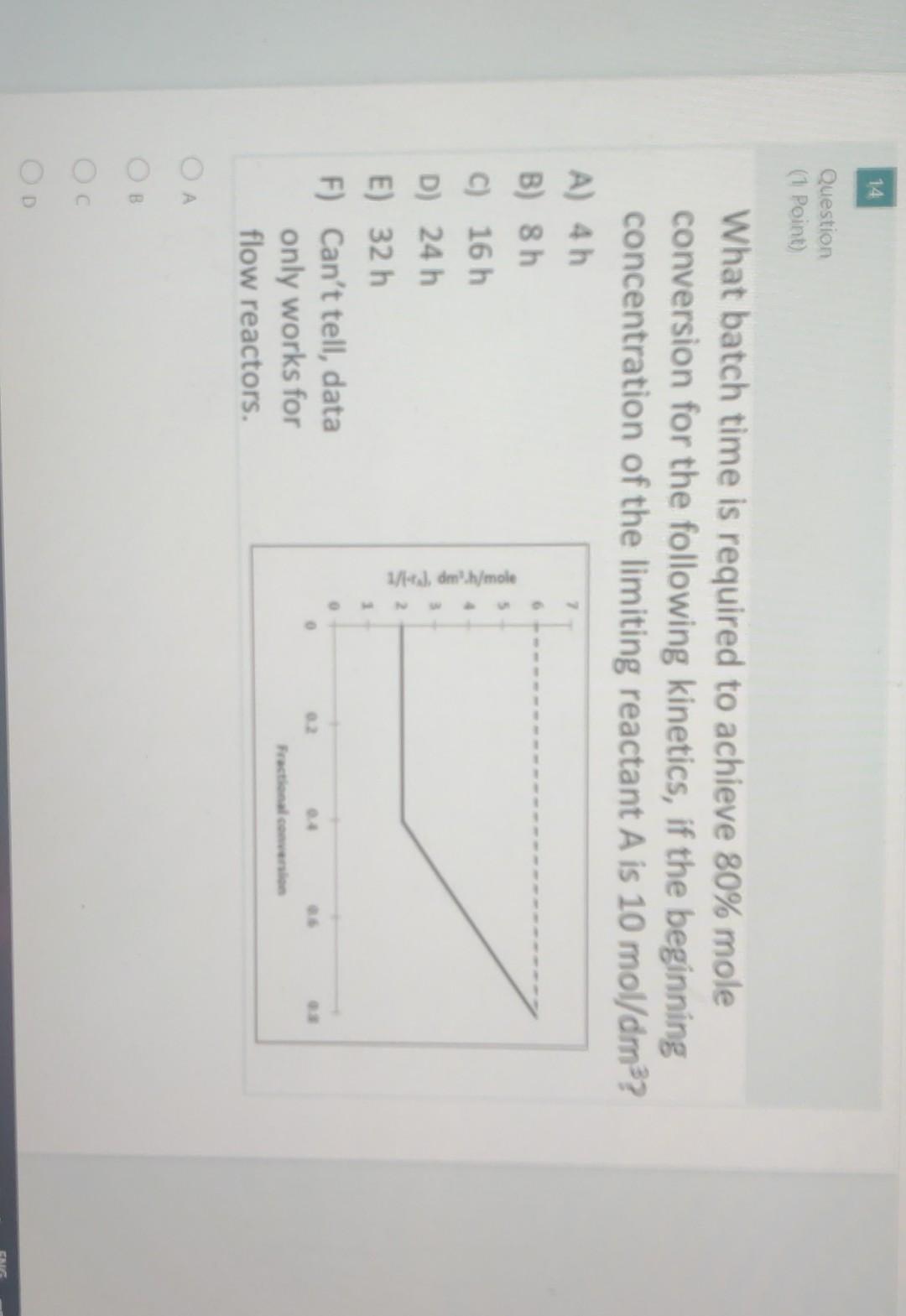
14 Question (1 Point) 5 What batch time is required to achieve 80% mole conversion for the following kinetics, if the beginning concentration of the limiting reactant A is 10 mol/dm3? A) 4h B) 8 h C) 16 h D) 24 h E) 32 h F) Can't tell, data only works for flow reactors. . 1/14), dm'h/mole Fractional convenio OB 0 0
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


