Answered step by step
Verified Expert Solution
Question
1 Approved Answer
*14.121. Elements of group 16 form hydrides with the generic formula H_(2)x . When gaseous H_(2)x is bubbled through a solution containing 0.3M hydrochloric acid,
*14.121. Elements of group 16 form hydrides with the generic\ formula
H_(2)x. When gaseous
H_(2)xis bubbled through a\ solution containing
0.3Mhydrochloric acid, the solution\ becomes saturated and
[H_(2)x]=0.1M. The following\ equilibria exist in this solution:\
H_(2)x(aq)+H_(2)O(l)Hx^(-)(aq)+H_(3)O^(+)(aq)K_(1)=8.3\\\\times 10^(-8)\ Hx^(-)(aq)+H_(2)O(l)x^(2-)(aq)+H_(3)O^(+)(aq)K_(2)=1\\\\times 10^(-14)\ Calculate the concentration of
x^(2-)in the solution.
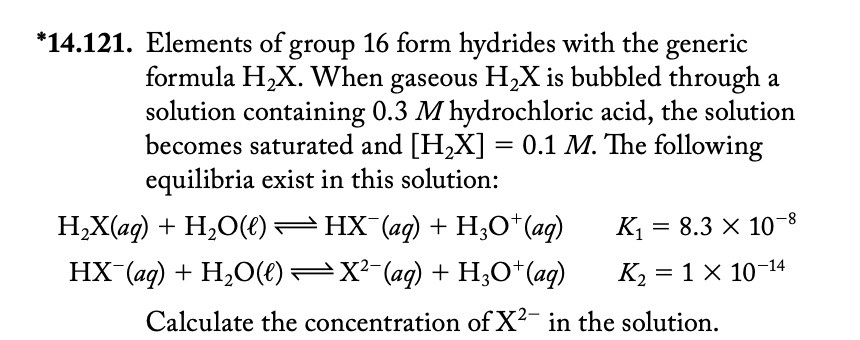
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


