Answered step by step
Verified Expert Solution
Question
1 Approved Answer
15 4) Consider the following molecule and draw (looking down the C1-C2 bond): a) The staggered conformation(s) of lowest energy (4 pts) b) The
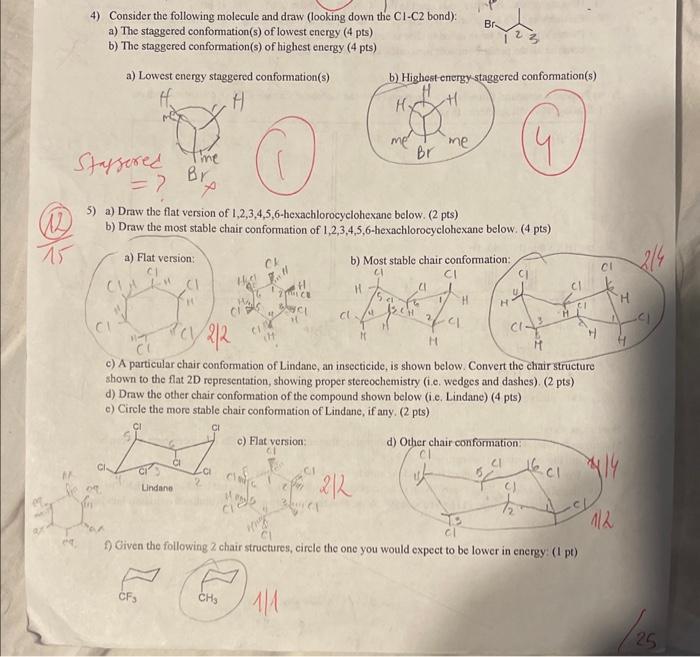
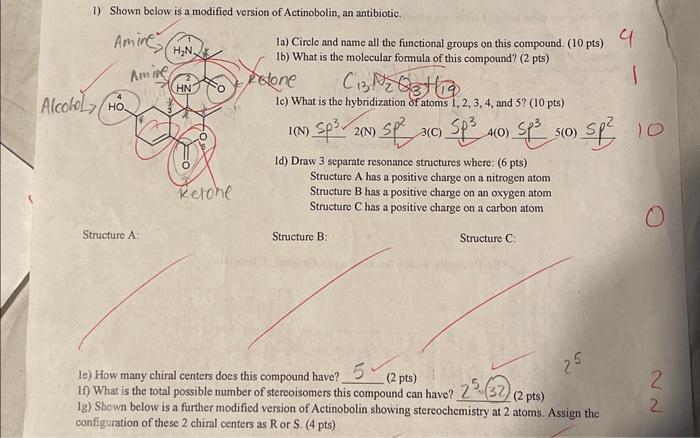
15 4) Consider the following molecule and draw (looking down the C1-C2 bond): a) The staggered conformation(s) of lowest energy (4 pts) b) The staggered conformation(s) of highest energy (4 pts) a) Lowest energy staggered conformation(s) H H Br b) Highest-energy-staggered conformation(s) Staggered Time Br =? P H me me Br 5) a) Draw the flat version of 1,2,3,4,5,6-hexachlorocyclohexane below. (2 pts) b) Draw the most stable chair conformation of 1,2,3,4,5,6-hexachlorocyclohexane below. (4 pts) a) Flat version: b) Most stable chair conformation: CI CK M CI H H 2/4 Cl H H H 2 H H M 4 c) A particular chair conformation of Lindane, an insecticide, is shown below. Convert the chair structure shown to the flat 2D representation, showing proper stereochemistry (i.e. wedges and dashes). (2 pts) d) Draw the other chair conformation of the compound shown below (i.e. Lindane) (4 pts) e) Circle the more stable chair conformation of Lindane, if any. (2 pts) a Untaine Cl c) Flat version: d) Other chair conformation: cl 212 f) Given the following 2 chair structures, circle the one you would expect to be lower in energy: (1 pt) 112 CF CHS 25 1) Shown below is a modified version of Actinobolin, an antibiotic. Amine Amine HN. HN Alcohol Ho 1a) Circle and name all the functional groups on this compound. (10 pts) 1b) What is the molecular formula of this compound? (2 pts) Felone C13239 lc) What is the hybridization of atoms 1, 2, 3, 4, and 5? (10 pts) Structure A: Ketone 2(N) 1) Sp 3(C) Sp3 4 4(0) Sp (0) Sp 10 1d) Draw 3 separate resonance structures where: (6 pts) Structure A has a positive charge on a nitrogen atom Structure B has a positive charge on an oxygen atom Structure C has a positive charge on a carbon atom Structure B: Structure C: 25 le) How many chiral centers does this compound have?, 5- (2 pts) If) What is the total possible number of stereoisomers this compound can have?, 25 (32) (2 pts) 1g) Shown below is a further modified version of Actinobolin showing stereochemistry at 2 atoms. Assign the configuration of these 2 chiral centers as R or S. (4 pts) 0 2 22
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


