Answered step by step
Verified Expert Solution
Question
1 Approved Answer
16. (4 pts) To produce food-grade phosphoric acid requires two steps. The first is a reaction of phosphorus with oxygen to produce gaseous diphosphorus
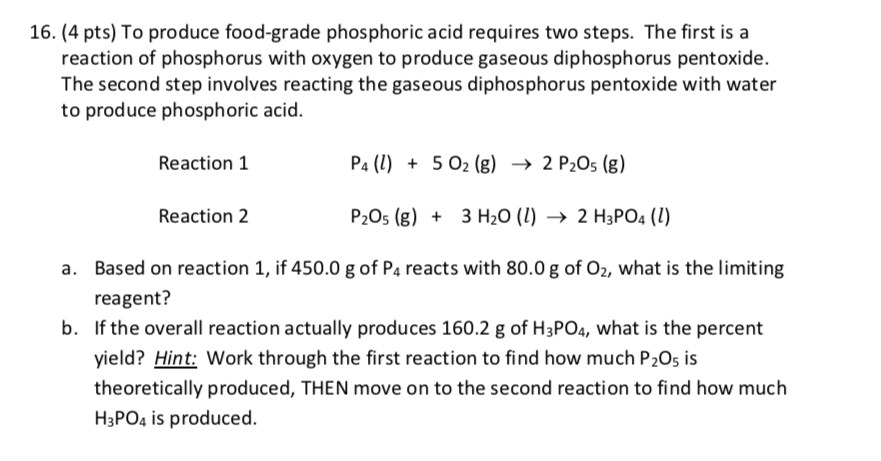
16. (4 pts) To produce food-grade phosphoric acid requires two steps. The first is a reaction of phosphorus with oxygen to produce gaseous diphosphorus pentoxide. The second step involves reacting the gaseous diphosphorus pentoxide with water to produce phosphoric acid. Reaction 1 Reaction 2 P4 (l) +502 (g) 2 P2O5 (g) P2O5 (g) +3 H2O (l) 2 H3PO4 (1) Based on reaction 1, if 450.0 g of P4 reacts with 80.0 g of O2, what is the limiting reagent? b. If the overall reaction actually produces 160.2 g of H3PO4, what is the percent yield? Hint: Work through the first reaction to find how much P2O5 is theoretically produced, THEN move on to the second reaction to find how much H3PO4 is produced.
Step by Step Solution
★★★★★
3.40 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1
a To determine the limiting reagent we need to compare the amounts of products that could be formed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


