Question
16. Consider a gas mixture whose apparent moleular weight is 33, initially at 3 bar and 300K, and occupying a vohme of 0.1 m.
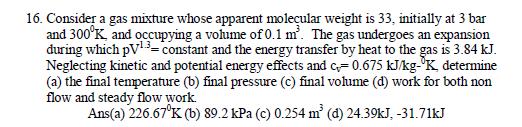
16. Consider a gas mixture whose apparent moleular weight is 33, initially at 3 bar and 300K, and occupying a vohme of 0.1 m. The gas undergoes an expansion during which pV13-constant and the energy transfer by heat to the gas is 3.84 kJ. Neglecting kinetic and potential energy effects and c0.675 kJkgK determine (a) the final temperature (b) final pressure (c) final volume (d) work for both non flow and steady flow work. Ans(a) 226.67K (b) 89.2 kPa (c) 0.254 m (d) 24.39kJ, -31.71kJ
Step by Step Solution
3.35 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physical Chemistry
Authors: Thomas Engel, Philip Reid
3rd edition
805338423, 080533842X, 978-0321812001
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



