Question: 16 Electrostatics The atom and electric charge Charging materials Insulators and conductors Induced charges Sparks and flashes The van de Graaff generator Digital sensors You

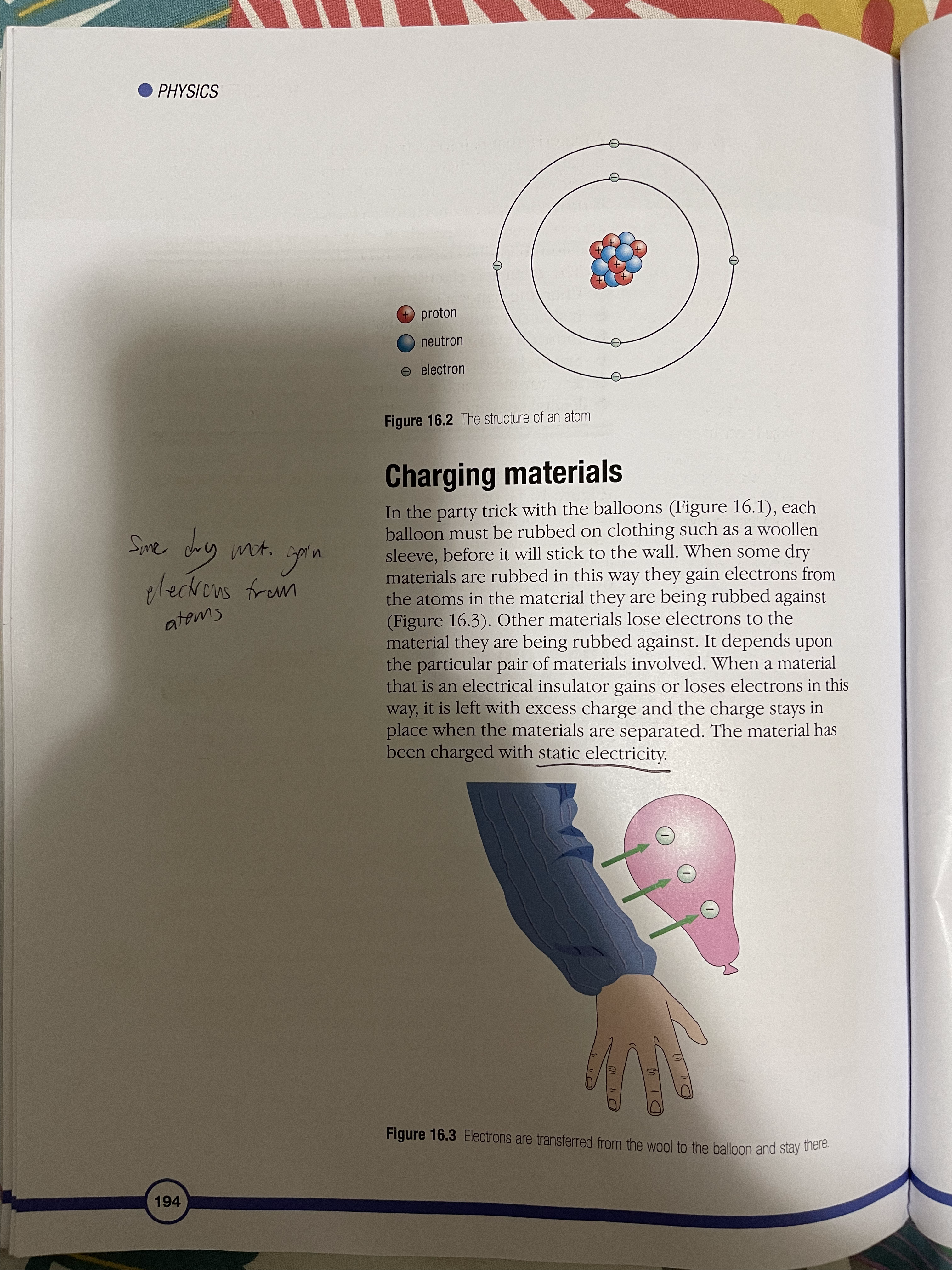
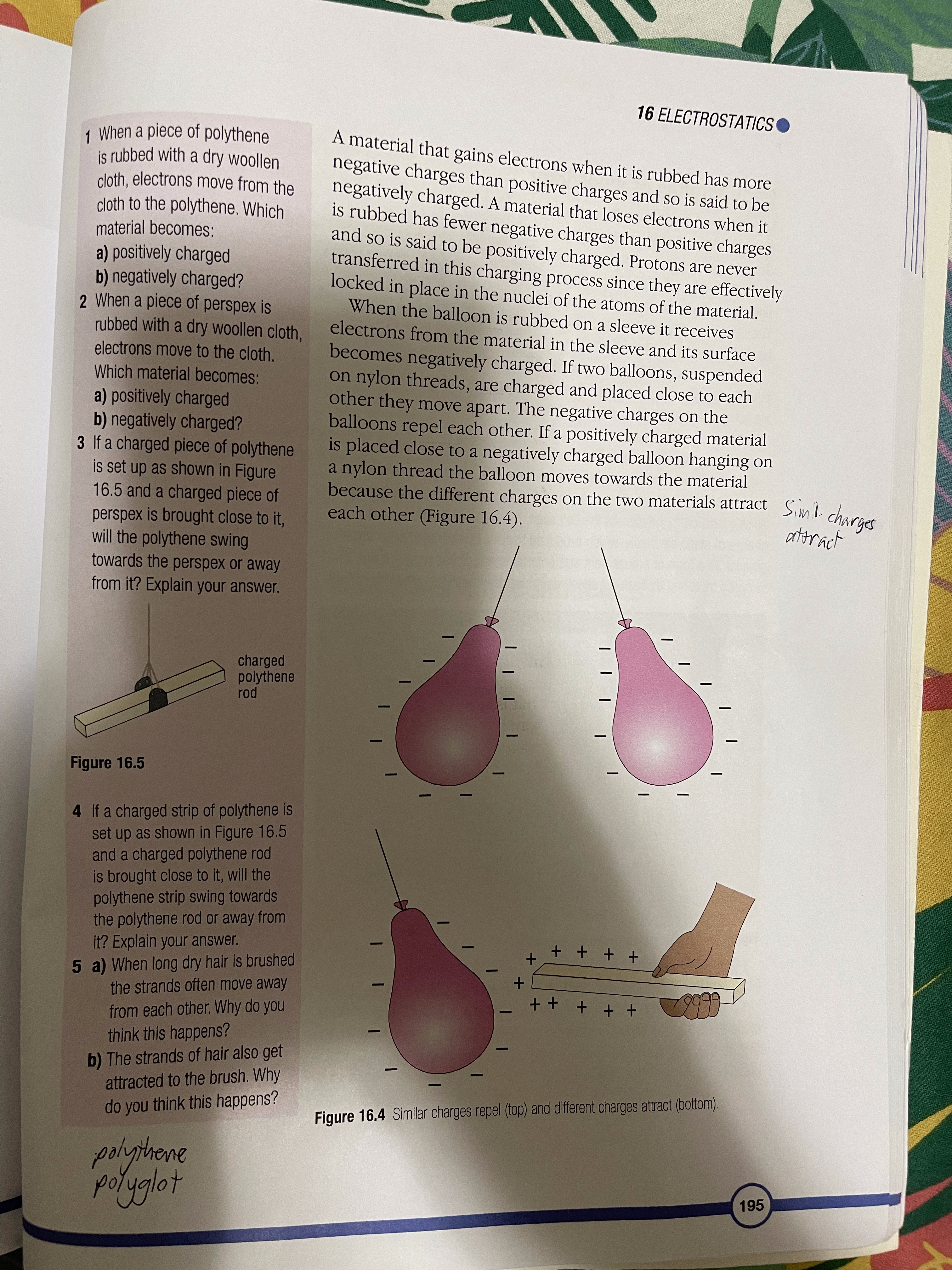



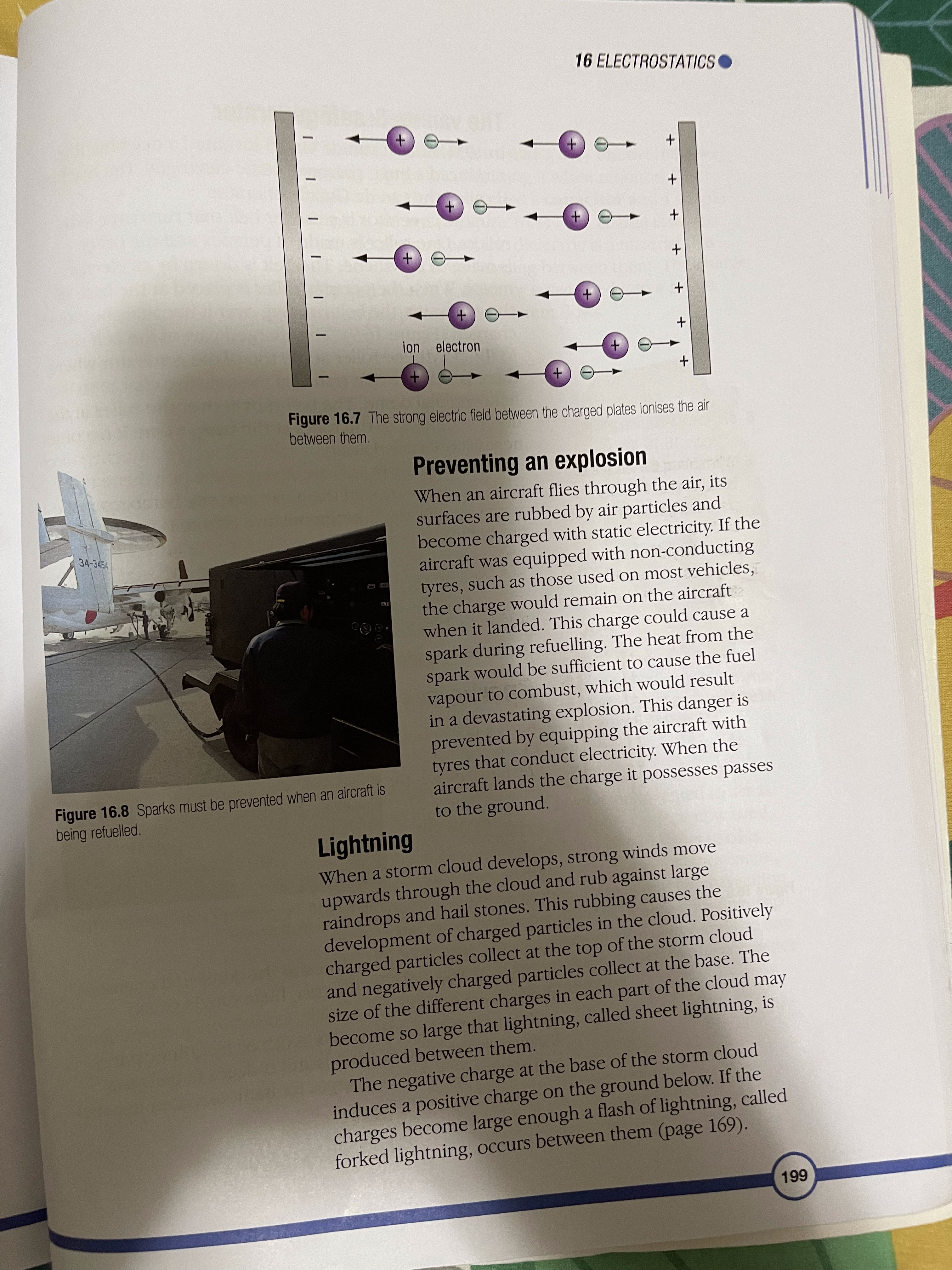
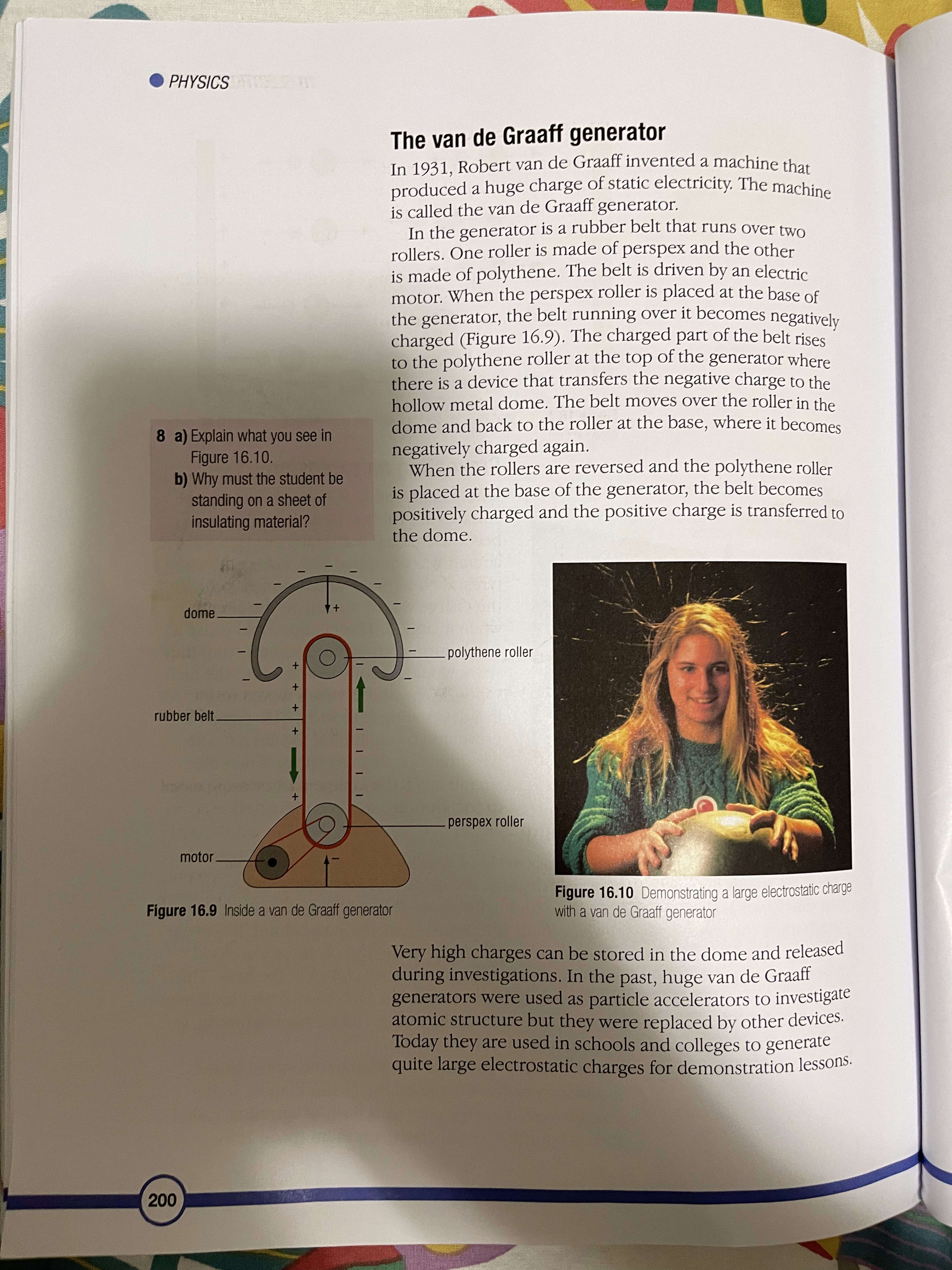


16 Electrostatics The atom and electric charge Charging materials Insulators and conductors Induced charges Sparks and flashes The van de Graaff generator Digital sensors You may have seen a balloon stuck to the wall or ceiling (Figure 16.1), or received a slight shock when touching a car door, or heard a crackle when taking off your jumper. To understand why these things happen, you have to think about the structure of the atom and the electric charges on the particles in it. The atom and electric charge An atom has a central nucleus surrounded by electrons and each electron carries a negative electric charge. In the nucleus are particles called protons and each proton carries a positive electric charge. Usually the number of positive charges carried by the protons is balanced by the number of negative charges carried by the electrons. For example, if an atom has six protons in its nucleus it has six electrons orbiting the nucleus. When the positive charges on the protons are balanced by the negative charges on the electrons in this way the atom is described as being neutral. As you can see in Figure 16.2, there are also particles called neutrons in the nucleus. They have no electric charge. Figure 16.1 These balloons are held in place by electrostatic forces 193PHYSICS + proton neutron electron Figure 16.2 The structure of an atom Charging materials In the party trick with the balloons (Figure 16.1), each Sme. dry mot. goin balloon must be rubbed on clothing such as a woollen sleeve, before it will stick to the wall. When some dry elections from materials are rubbed in this way they gain electrons from the atoms in the material they are being rubbed against atoms (Figure 16.3). Other materials lose electrons to the material they are being rubbed against. It depends upon the particular pair of materials involved. When a material that is an electrical insulator gains or loses electrons in this way, it is left with excess charge and the charge stays in place when the materials are separated. The material has been charged with static electricity. Figure 16.3 Electrons are transferred from the wool to the balloon and stay there. 19416 ELECTROSTATICS 1 When a piece of polythene is rubbed with a dry woollen A material that gains electrons when it is rubbed has more cloth, electrons move from the negative charges than positive charges and so is said to be cloth to the polythene. Which negatively charged. A material that loses electrons when it material becomes: is rubbed has fewer negative charges than positive charges a) positively charged and so is said to be positively charged. Protons are never b) negatively charged? transferred in this charging process since they are effectively locked in place in the nuclei of the atoms of the material. 2 When a piece of perspex is When the balloon is rubbed on a sleeve it receives rubbed with a dry woollen cloth, electrons from the material in the sleeve and its surface electrons move to the cloth. becomes negatively charged. If two balloons, suspended Which material becomes: on nylon threads, are charged and placed close to each a) positively charged other they move apart. The negative charges on the b) negatively charged? balloons repel each other. If a positively charged material 3 If a charged piece of polythene is placed close to a negatively charged balloon hanging on is set up as shown in Figure a nylon thread the balloon moves towards the material 16.5 and a charged piece of because the different charges on the two materials attract Simil charges perspex is brought close to it, each other (Figure 16.4). attract will the polythene swing towards the perspex or away from it? Explain your answer. charged polythene rod Figure 16.5 4 If a charged strip of polythene is set up as shown in Figure 16.5 and a charged polythene rod is brought close to it, will the polythene strip swing towards the polythene rod or away from it? Explain your answer. + + + + + 5 a) When long dry hair is brushed + the strands often move away + + + + + from each other. Why do you think this happens? b) The strands of hair also get attracted to the brush. Why do you think this happens? Figure 16.4 Similar charges repel (top) and different charges attract (bottom). polythere polyglot 195PHYSICS Early studies of electricity For millions of years, there have been certain kinds of trees producing sap that turns to a clear yellow fossilised substance called amber. In Ancient Greece, amber was used in items of jewellery. Thales (624-546 BCE), the Electron earliest Greek 'scientific' philosopher, noticed that if amber was rubbed it developed the power to pick up small objects like dust, straw and feathers. The Greek word for amber is elektron so, much later, its attractive power became known as electricity. William Gilbert (1544-1603), an English scientist, discovered that a few other materials, such as certain gemstones and rock crystal, could also attract small objects when they were rubbed. He called these materials 'electrics'. Van A German scientist called Otto von Guericke (1602-1686) used an electric' called sulfur to make a machine that could generate sparks. He made the sulfur into a ball and attached it to an axle that could be turned quickly by a crank handle. As the ball span, it was rubbed and built up a charge of static electricity, which produced sparks. Electric machines became der otto von Guericke popular as a form of amusement and entertainment. Some people made their living by travelling through European countries, demonstrating their machines. 1 Where did the word electricity' come from? 2 Why do you think electric Figure A An electric machine built in about 1762 machines amused people? Stephen Gray (1696-1736), another English scientist, investigated 3 How was the evidence electricity by rubbing a glass tube that had corks in the ends, and provided by Thales discovered that the corks became charged with static electricity even observation used though they had not been touched. He had discovered that electricity by Gilbert to extend the behaves as if it can flow like a liquid. knowledge of electricity? 1962 fluids of electricity 16 ELECTROSTATICS Charles Du Fay (1698-1739), a French scientist, repeated Gray's experiments and extended them by comparing the way in which the 4 How was the evidence objects were charged with electricity. He discovered that if he charged provided by Gray's a cork ball using a glass rod that had been rubbed, it was attracted to a work used by Du Fay cork ball that had been charged using sealing wax that had been rubbed. to secure and extend the He also discovered that two cork balls that had both been charged by knowledge of electricity? either the rubbed glass or by the rubbed sealing wax repelled each other. 5 What creative thoughts did From his investigations he believed that electricity was made from two Franklin apply to Du Fay's work different fluids. They were called 'vitreous electricity' (from rubbing glass) to refine ideas about electricity? and 'resinous electricity' (from rubbing sealing wax). Benjamin Franklin (1706-1790), an American statesman and scientist, 6 a) How was Franklin's idea of refined Du Fay's idea of two electrical fluids by suggesting that when electric charge similar to the substances were charged they received either too much fluid and became ideas that we use today? positively charged or they had some fluid taken away and became b) How was his idea of electric negatively charged. charge different to those we use today? ke Insulators and conductors A material that can become charged with static electricity is called an insulator. If electrons are added to the material they stay in place and the insulator is negatively charged. If electrons are removed from the material more electrons do not flow into the material and it remains positively charged. A metal cannot be charged with electricity by rubbing, in the way that an insulating material can, because electrons flow easily through metals. A material through which Conductus body electrons can flow is called a conductor. The human body Ex: hum. is a very good conductor of electricity. Induced charges If a material has an electric charge, it can make or 'induce' an electric charge on the surface of a material close by without touching the material. For example, if a piece of plastic, such as a pen, is rubbed and held above a tiny piece of paper, the positive charge on the plastic draws electrons to the surface of the paper nearest the plastic. This makes the uppermost surface of the paper negatively charged. When the pen is brought very close to the paper the force of attraction between the two surfaces is strong enough to overcome the weight of the paper and the paper springs up to the surface of the pen. 197PHYSICS Figure 16.6 The charged pen induces charges on the surfaces of the paper scraps. The underside of the paper is left with a positive charge but since this is further away from the pen the force of repulsion it experiences is weaker than the attractive force 6 When a negative charge is and the paper is held. induced on one surface of a In a similar way a charged balloon induces an opposite piece of paper, what is induced charge on the surface of a wall it is brought close to. When on the other surface of the the balloon touches the wall, the force of attraction paper? Why does this happen? between the two surfaces is greater than the weight of the 7 Why does a rubbed balloon balloon and the air inside it, so the balloon sticks to the stick to the wall? wall (Figure 16.1). This way of charging a material without touching it is called charging by induction. Sparks and flashes Air is a poor conductor of electricity but if the size of the charge on two oppositely charged surfaces is very large the air between them may conduct electricity as a spark or a flash, like a flash of lightning. This happens when the molecules in the air are split. They form negatively charged electrons and positively charged ions. The electrons move towards the positively charged surface and ions move towards the negative surface (Figure 16.7). As the electrons move they collide with other molecules in the air and split them. The ions and electrons from these molecules also move towards the charged surfaces and split more molecules as they go. This process occurs very quickly and produces a spark. When the charged particles in the air meet the charged surfaces, the positive and negative charges cancel each other out and the surfaces lose their charge. They are said to have been discharged. 19816 ELECTROSTATICS. +) (+ + +) (+ + (+) + +) A- + ion electron Figure 16.7 The strong electric field between the charged plates ionises the air between them. Preventing an explosion When an aircraft flies through the air, its surfaces are rubbed by air particles and 34-345 become charged with static electricity. If the aircraft was equipped with non-conducting tyres, such as those used on most vehicles, the charge would remain on the aircraft when it landed. This charge could cause a spark during refuelling. The heat from the spark would be sufficient to cause the fuel vapour to combust, which would result in a devastating explosion. This danger is prevented by equipping the aircraft with tyres that conduct electricity. When the Figure 16.8 Sparks must be prevented when an aircraft is aircraft lands the charge it possesses passes being refuelled. to the ground. Lightning When a storm cloud develops, strong winds move upwards through the cloud and rub against large raindrops and hail stones. This rubbing causes the development of charged particles in the cloud. Positively charged particles collect at the top of the storm cloud and negatively charged particles collect at the base. The size of the different charges in each part of the cloud may become so large that lightning, called sheet lightning, is produced between them. The negative charge at the base of the storm cloud induces a positive charge on the ground below. If the charges become large enough a flash of lightning, called forked lightning, occurs between them (page 169). 199PHYSICS The van de Graaff generator In 1931, Robert van de Graaff invented a machine that produced a huge charge of static electricity. The machine is called the van de Graaff generator. In the generator is a rubber belt that runs over two rollers. One roller is made of perspex and the other is made of polythene. The belt is driven by an electric motor. When the perspex roller is placed at the base of the generator, the belt running over it becomes negatively charged (Figure 16.9). The charged part of the belt rises to the polythene roller at the top of the generator where there is a device that transfers the negative charge to the hollow metal dome. The belt moves over the roller in the 8 a) Explain what you see in dome and back to the roller at the base, where it becomes Figure 16.10. negatively charged again. b) Why must the student be When the rollers are reversed and the polythene roller standing on a sheet of is placed at the base of the generator, the belt becomes insulating material? positively charged and the positive charge is transferred to the dome. dome - polythene roller rubber belt. perspex roller motor Figure 16.10 Demonstrating a large electrostatic charge Figure 16.9 Inside a van de Graaff generator with a van de Graaff generator Very high charges can be stored in the dome and released during investigations. In the past, huge van de Graaff generators were used as particle accelerators to investigate atomic structure but they were replaced by other devices. Today they are used in schools and colleges to generate quite large electrostatic charges for demonstration lessons. 20016 ELECTROSTATICS Digital sensors As scientists studied electrostatics they discovered a way of storing charge and releasing it when required. The charge-storing device is called a capacitor and a simple example is shown in Figure 16.11. The charge is stored on the metal plates and the dielectric is a material that stops the charge from crossing between them. The charge is released when a conductor is switched into a circuit between the plates and a current flows. dielectric connections to plates AtDe (AND ACTION XZ OL MACHI ASE 315 15.4 Inc Q O metal plates Figure 16.11 A simple capacitor Figure 16.12 Touch screen technology would not have been possible without the development of the capacitor. Since the development of the simple capacitor using metal plates tiny, electronic devices have been invented to act as switches, conductors and capacitors. These are now used in all kinds of electronic apparatus, from sensors used in laboratories to detect changes in light, pH and movement, to the touch screens on mobile phones, satellite navigation devices and other computer displays, where your fingertip and the screen act like the plates of a capacitor and the tiny difference in charge between them is used by the computer in the appliance to trigger a response. 201PHYSICS SUMMARY An atom has two kinds of electrically charged particles. They are protons and electrons (see page 193). Static electricity may be generated by rubbing insulators (see page 194). Charged materials can have either a positive or a negative charge (see page 195). Materials that hold charges of static electricity are called insulators. Materials that allow electricity to pass through them are called conductors (see page 197). A material can become charged by induction (see page 197). Static electricity can be discharged through the air as a spark or a flash (see page 198). The van de Graaff generator can produce large charges and can be used to study the effects created by static electricity (see page 200). Electrostatic devices are used in digital sensors (see page 201). End of chapter question How could you use your knowledge of the structure of the atom to explain how a plastic pen that has been rubbed can pick up tiny pieces of paper? 202
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


