Question
1.7 g of helium at an initial temperature of 350 K interacts thermally with 8.6 g of oxygen at an initial temperature of 620
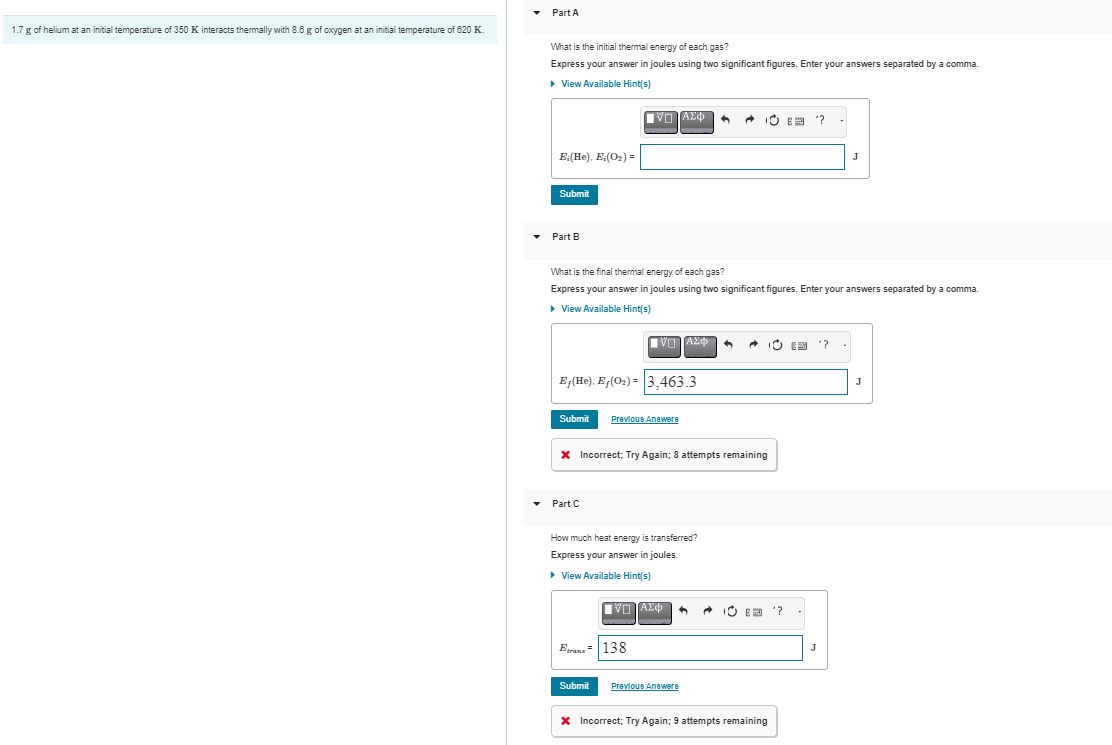
1.7 g of helium at an initial temperature of 350 K interacts thermally with 8.6 g of oxygen at an initial temperature of 620 K Part A What is the initial thermal energy of each gas? Express your answer in joules using two significant figures. Enter your answers separated by a comma. View Available Hint(s) E:(He). E;(O2)= Submit Part B '? J What is the final thermal energy of each gas? Express your answer in joules using two significant figures. Enter your answers separated by a comma. View Available Hint(s) E,(He). E(O2)=3,463.3 Submit Previous Answere * Incorrect; Try Again; 8 attempts remaining Part C How much heat energy is transferred? Express your answer in joules. View Available Hint(s) Etrana 138 '? Submit Previous Answer * Incorrect; Try Again; 9 attempts remaining J '? J
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics for Scientists and Engineers A Strategic Approach with Modern Physics
Authors: Randall D. Knight
4th edition
978-0134092508, 134092503, 133942651, 978-0133942651
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



