Answered step by step
Verified Expert Solution
Question
1 Approved Answer
18, 31,32 please answer all the questions 1.08490 g of octane (molar mass = 114 g/mol) was burnt to heat water in a calorimetry experiment.
18, 31,32 please answer all the questions 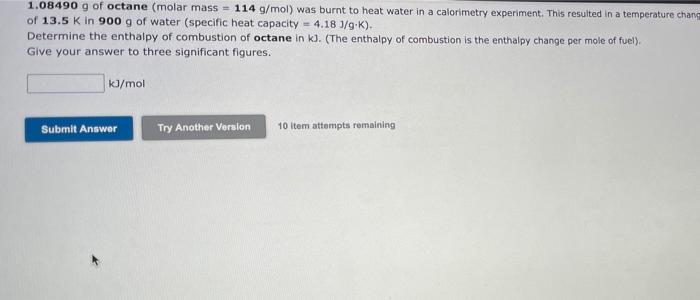
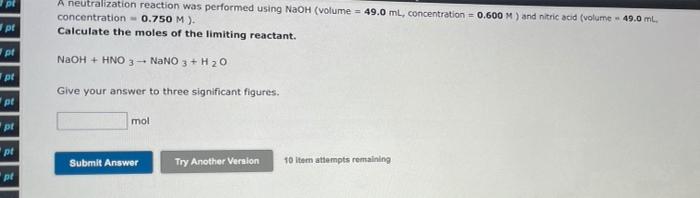
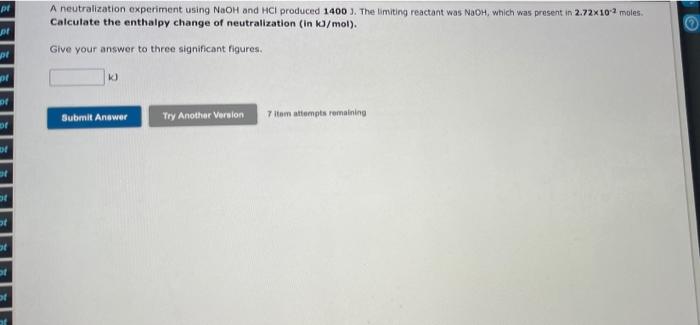
1.08490 g of octane (molar mass = 114 g/mol) was burnt to heat water in a calorimetry experiment. This resulted in a temperature chane of 13.5 K in 900 g of water (specific heat capacity = 4.18 J/g.K). Determine the enthalpy of combustion of octane in k. (The enthalpy of combustion is the enthalpy change per mole of fuel). Give your answer to three significant figures. kJ/mol Submit Answer Try Another Version 10 Item attempts remaining A neutralization reaction was performed using NaOH (volume = 49.0 ml, concentration = 0.600 M) and nitric acid (volume 49.0 ml concentration - 0.750 M). Calculate the moles of the limiting reactant. pe pi NaOH + HNO 3 - NaNO3 + H2O pl Give your answer to three significant figures. pl mol pl pl Submit Answer Try Another Version 10 ltem attempts remaining pl A neutralization experiment using NaOH and HCI produced 1400 ). The limiting reactant was NaOH, which was present in 2.72x102 moles Calculate the enthalpy change of neutralization (in kJ/mol). pl Give your answer to three significant figures. Submit Answer Try Another Version 7 tom attempts remaining at af 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


