Question
1.8-1. For the system considered in Example 1, calculate the energy of the state with P = 5 x 104 Pa and V =
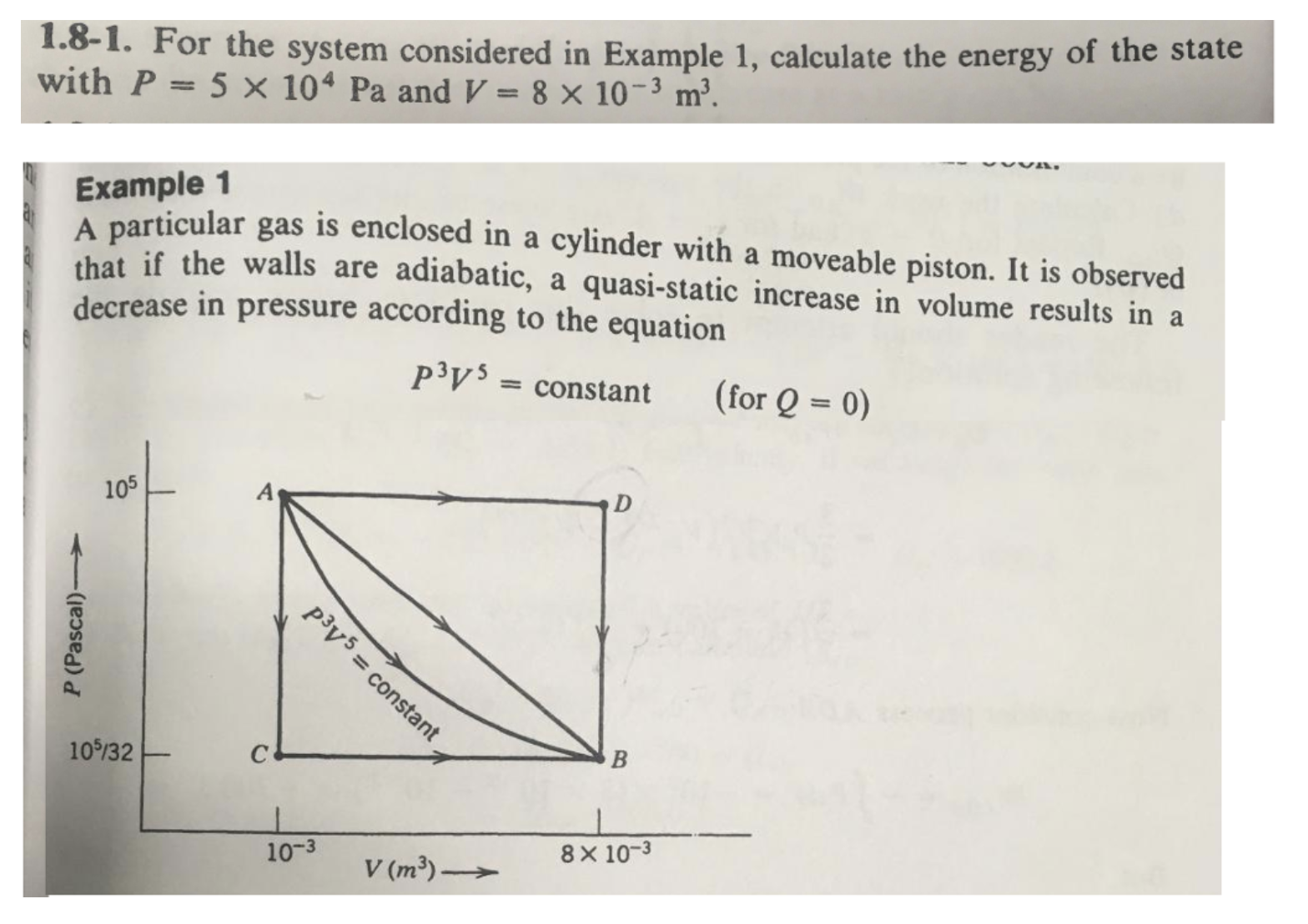
1.8-1. For the system considered in Example 1, calculate the energy of the state with P = 5 x 104 Pa and V = 8 10-3 m. Example 1 A particular gas is enclosed in a cylinder with a moveable piston. It is observed that if the walls are adiabatic, a quasi-static increase in volume results in a decrease in pressure according to the equation py5 = constant (for Q = 0) P (Pascal)- 105 105/32 py5 = constant 10-3 V (m)- B D 8 10-3
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
College Physics
Authors: OpenStax
2nd Edition
171147083X, 978-1711470832
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



