Answered step by step
Verified Expert Solution
Question
1 Approved Answer
18.31 Oxygen (O) has a molar mass of 32.0 g/mol. What is (a) the average translational kinetic energy of an oxygen molecule at a tempera-
18.31 Oxygen (O) has a molar mass of 32.0 g/mol. What is (a) the average translational kinetic energy of an oxygen molecule at a tempera- ture of 300 K; (b) the average value of the square of its speed; (c) the root- mean-square speed; (d) the momentum of an oxygen molecule traveling at this speed? (e) Suppose an oxygen molecule traveling at this speed bounces back and forth between opposite sides of a cubical vessel 0.10 m on a side. What is the average force the molecule exerts on one of the walls of the container? (Assume that the molecule's velocity is perpendic- ular to the two sides that it strikes.) (f) What is the average force per unit area? (g) How many oxygen molecules traveling at this speed are neces- sary to produce an average pressure of 1 atm? (h) Compute the number of oxygen molecules that are contained in a vessel of this size at 300 K and atmospheric pressure. (i) Your answer for part (h) should be three times as large as the answer for part (g). Where does this discrepancy arise? ..
coukd you help me with part e and forward?
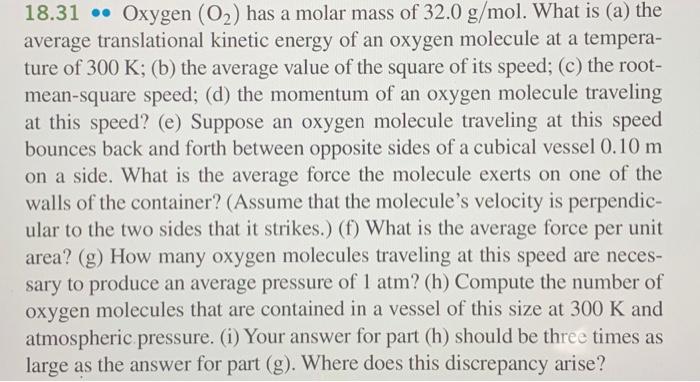
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


