Answered step by step
Verified Expert Solution
Question
1 Approved Answer
19. Consider a container with a piston containing 2.0 moles of a monatomic ideal gas with initial conditions of V=6.0 Land P=2.0atm at 300K. If
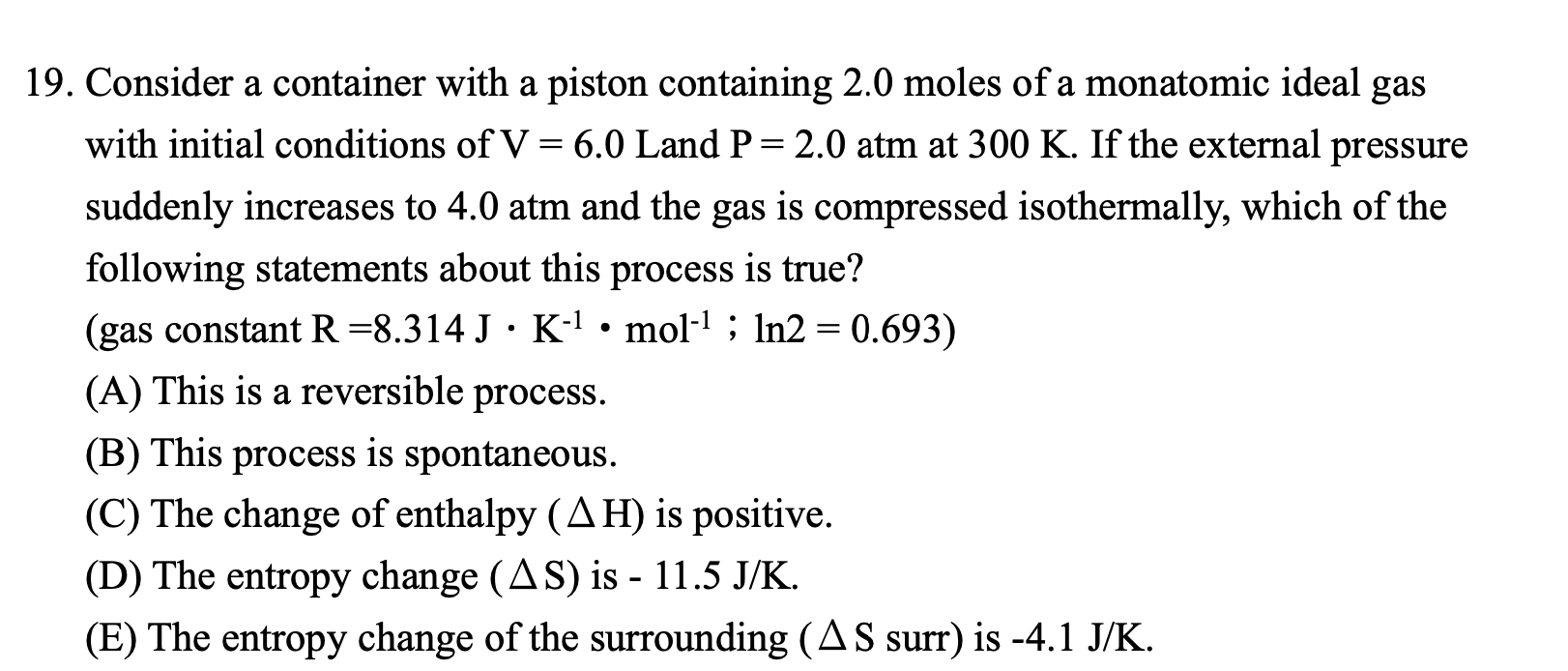 19. Consider a container with a piston containing 2.0 moles of a monatomic ideal gas with initial conditions of V=6.0 Land P=2.0atm at 300K. If the external pressure suddenly increases to 4.0atm and the gas is compressed isothermally, which of the following statements about this process is true? (gas constant R=8.314JK1mol1;ln2=0.693 ) (A) This is a reversible process. (B) This process is spontaneous. (C) The change of enthalpy (H) is positive. (D) The entropy change (S) is 11.5J/K. (E) The entropy change of the surrounding ( S surr) is 4.1J/K
19. Consider a container with a piston containing 2.0 moles of a monatomic ideal gas with initial conditions of V=6.0 Land P=2.0atm at 300K. If the external pressure suddenly increases to 4.0atm and the gas is compressed isothermally, which of the following statements about this process is true? (gas constant R=8.314JK1mol1;ln2=0.693 ) (A) This is a reversible process. (B) This process is spontaneous. (C) The change of enthalpy (H) is positive. (D) The entropy change (S) is 11.5J/K. (E) The entropy change of the surrounding ( S surr) is 4.1J/K Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


