Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1.95 - ). mperature, calculate 1/T (in units of kelvins), and in show examples of your work to the right and below the table. Temperature
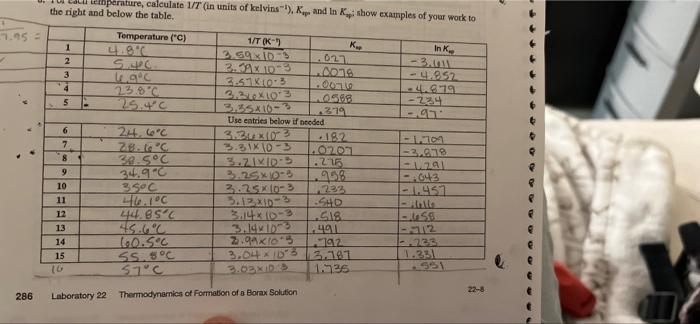
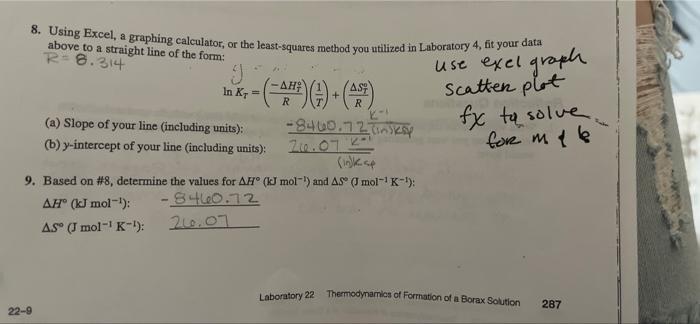


 1.95 - ). mperature, calculate 1/T (in units of kelvins), and in show examples of your work to the right and below the table. Temperature (C) 1/T (K) 1 K 4.C Ink 2.59 XDR 2 02 Sec. -3 3.3AX09 3 C -4.852 4 3.5 1103 007 23.8C 3.24X102 -4.819 5 PO588 25.4C 224 3.55x10-3 1319 Use entries below if needed - 6 24.6C 3.2002 192 7 ZB.6C - 1.100 3.31x10 - 3 8 -2.979 38.5C 3.ZIKIDES .215 -111 9 34.90 3.25x10-3 998 043 10 3 goc 3.25*10-3 222 -1.451 11 46.100 3.12.XID-3 SHD 12 44.85C .SIS US 13 45.6C 3.1419 491 12 14 60.5C 2.9 x 103 192 15 ss sC 3.04.15 3.11 1.331 10 S7C 3.02 XD 1.236 22-8 Laboratory 22 Thermodynamics of Formation of a Borax Solution 286 8. Using Excel, a graphing calculator, or the least-squares method you utilized in Laboratory 4, fit your data above to a straight line of the form: R-8.314 9 -(-983) ) + () -8460.72 linksy + R R use exel graph Scatter plot fx to solve , for me (a) Slope of your line (including units): (b) y-intercept of your line (including units): 21.07.2 9. Based on #8, determine the values for AN (kJ mol-) and As mol-'K-1) A(kJ mol-!): - 8460.72 AS" (J mol-'K-1): 216.07 Laboratory 22 Thermodynamics of Formation of a Borax Solution 287 22-9 2. The literature values for enthalpy and entropy of the dissolution of borax in water are 110 kJ mol- and 380 J mol- k-1, respectively, (a) Determine the percent error in your experimentally determined values (#9). - (b) What could be the possible source(s) of error leading to your results? Calculations are wrong Titration done incorrectly Not the exact temperature Solids left behind 3. Based on your data, (a) Does the solubility of borax in water increase/decrease/stay the same as the temperature is increased? increase . of hon in water an exothermic or endothermic process? (c) Does your answer in part (a) make sense when considering your value for AH"? Explain. 4. Clearly explain what your value of AS means (i.e., is your experimentally determined value consistent with what you would predict based on Reaction 1?). 5. Was there a large degree of variability in (borate] between different groups measuring the same approximate temperature? If so, what might lead to this? In most groups, what might read to this is not being careful with titrations, having not S 6. Suppose a solution was made with the following concentrations at 30C. [borate) -0,25 M, [Na*) = 0.35 M. (a) Using the results of your experiment, determine whether this system is at equilibrium or, if not, in which direction it will spontaneously proceed to reach equilibrium. (b) Will precipitate be formed during part (a)? Connection Based on your experience in this lab, draw a connection to something in your everyday life of the world around you (something not mentioned in the background section): changes like when it rains, Snows, Hails
1.95 - ). mperature, calculate 1/T (in units of kelvins), and in show examples of your work to the right and below the table. Temperature (C) 1/T (K) 1 K 4.C Ink 2.59 XDR 2 02 Sec. -3 3.3AX09 3 C -4.852 4 3.5 1103 007 23.8C 3.24X102 -4.819 5 PO588 25.4C 224 3.55x10-3 1319 Use entries below if needed - 6 24.6C 3.2002 192 7 ZB.6C - 1.100 3.31x10 - 3 8 -2.979 38.5C 3.ZIKIDES .215 -111 9 34.90 3.25x10-3 998 043 10 3 goc 3.25*10-3 222 -1.451 11 46.100 3.12.XID-3 SHD 12 44.85C .SIS US 13 45.6C 3.1419 491 12 14 60.5C 2.9 x 103 192 15 ss sC 3.04.15 3.11 1.331 10 S7C 3.02 XD 1.236 22-8 Laboratory 22 Thermodynamics of Formation of a Borax Solution 286 8. Using Excel, a graphing calculator, or the least-squares method you utilized in Laboratory 4, fit your data above to a straight line of the form: R-8.314 9 -(-983) ) + () -8460.72 linksy + R R use exel graph Scatter plot fx to solve , for me (a) Slope of your line (including units): (b) y-intercept of your line (including units): 21.07.2 9. Based on #8, determine the values for AN (kJ mol-) and As mol-'K-1) A(kJ mol-!): - 8460.72 AS" (J mol-'K-1): 216.07 Laboratory 22 Thermodynamics of Formation of a Borax Solution 287 22-9 2. The literature values for enthalpy and entropy of the dissolution of borax in water are 110 kJ mol- and 380 J mol- k-1, respectively, (a) Determine the percent error in your experimentally determined values (#9). - (b) What could be the possible source(s) of error leading to your results? Calculations are wrong Titration done incorrectly Not the exact temperature Solids left behind 3. Based on your data, (a) Does the solubility of borax in water increase/decrease/stay the same as the temperature is increased? increase . of hon in water an exothermic or endothermic process? (c) Does your answer in part (a) make sense when considering your value for AH"? Explain. 4. Clearly explain what your value of AS means (i.e., is your experimentally determined value consistent with what you would predict based on Reaction 1?). 5. Was there a large degree of variability in (borate] between different groups measuring the same approximate temperature? If so, what might lead to this? In most groups, what might read to this is not being careful with titrations, having not S 6. Suppose a solution was made with the following concentrations at 30C. [borate) -0,25 M, [Na*) = 0.35 M. (a) Using the results of your experiment, determine whether this system is at equilibrium or, if not, in which direction it will spontaneously proceed to reach equilibrium. (b) Will precipitate be formed during part (a)? Connection Based on your experience in this lab, draw a connection to something in your everyday life of the world around you (something not mentioned in the background section): changes like when it rains, Snows, Hails

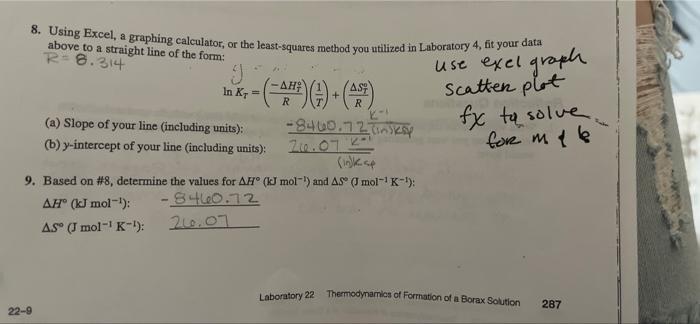



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


