Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1.a; 1, b; 1.c --All obey the Lewis octet rule. 2.a; 2, b; 2.c --None obey the Lewis octet rule. 3.a; 3, b; 3.c --No
1.a; 1, b; 1.c --All obey the Lewis octet rule.
2.a; 2, b; 2.c --None obey the Lewis octet rule.
3.a; 3, b; 3.c --No adherence to the Lewis octet rule is indicated.
4.a; 4, b; 4.c --For molecules or molecular ions with two or more atoms considered as central atoms, consider each atom separately in the analysis, according to Table D3.3.
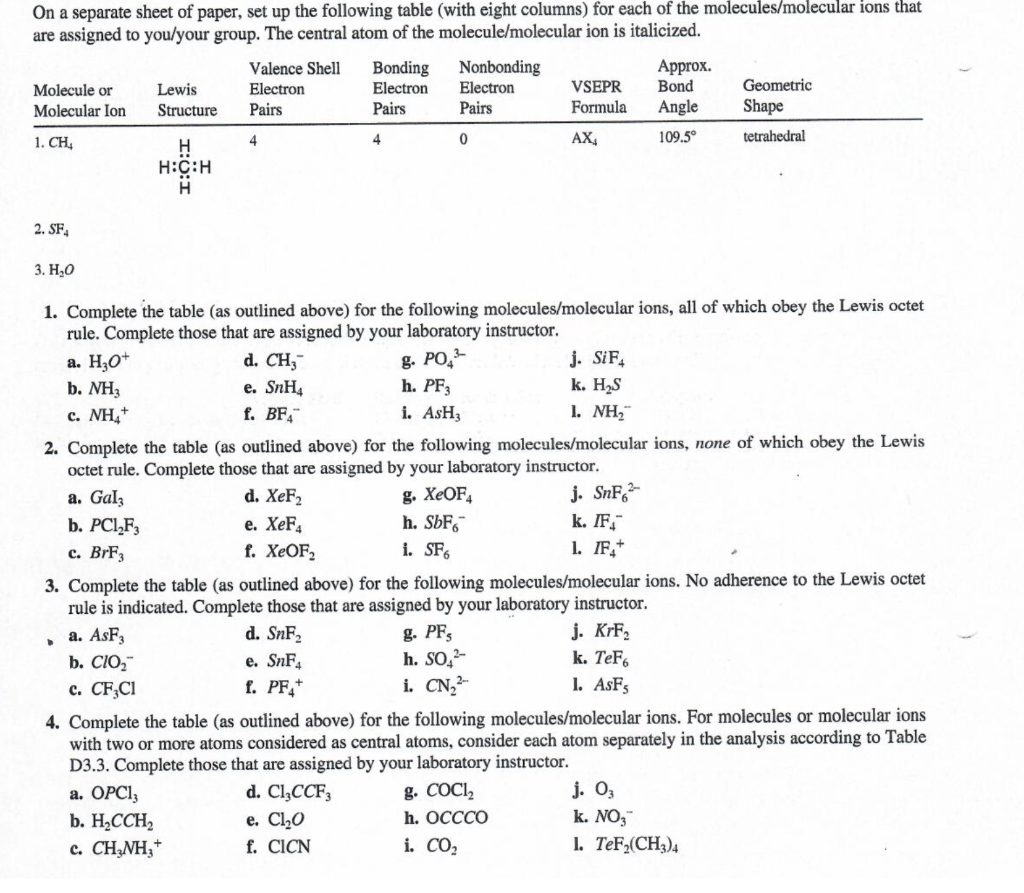
On a separate sheet of paper, set up the following table (with eight columns) for each of the molecules/molecular ions that are assigned to you/your group. The central atom of the molecule/molecular ion is italicized. 2. SF4 3. H2O 1. Complete the table (as outlined above) for the following molecules/molecular ions, all of which obey the Lewis octet rule. Complete those that are assigned by your laboratory instructor. a. H3O+ d. CH3 g. PO43 j. SiF4 b. NH3 e. SnH4 h. PF3 k. H2S c. NH4+ f. BF4 i. AsH3 l. NH2 2. Complete the table (as outlined above) for the following molecules/molecular ions, none of which obey the Lewis octet rule. Complete those that are assigned by your laboratory instructor. a. GaI3 d. XeF2 g. XeOF4 j. SnF62 b. PCl2F3 e. XeF4 h. SbF6 k. F4 c. BrF3 f. XeOFOF2 i. SF6 1. IF4+ 3. Complete the table (as outlined above) for the following molecules/molecular ions. No adherence to the Lewis octet rule is indicated. Complete those that are assigned by your laboratory instructor. a. AsF3 d. SnF2 g. PF5 j. KrF2 b. ClO2 e. SnF4 h. SO42 k. TeFF6 c. CF3Cl f. PF4+ i. CN22 l. AsF5 4. Complete the table (as outlined above) for the following molecules/molecular ions. For molecules or molecular ions with two or more atoms considered as central atoms, consider each atom separately in the analysis according to Table D3.3. Complete those that are assigned by your laboratory instructor. a. OPCl3 d. Cl3CCF3 g. COCl2 j. O3 b. H2CCH2 e. Cl2O h. OCCCO k. NO3 c. CH3NH3+ f. ClCN i. CO2 l. TeF2(CH3)4Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


