Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1.a) In the electrolysis of water, how long will it take to produce 24 L of H gas at 23 atm and 26 C
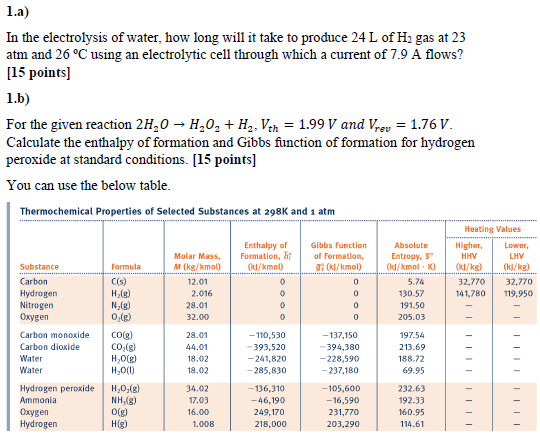
1.a) In the electrolysis of water, how long will it take to produce 24 L of H gas at 23 atm and 26 C using an electrolytic cell through which a current of 7.9 A flows? [15 points] 1.b) For the given reaction 2HO HO + H, Vth = 1.99 V and Vrev = 1.76 V. Calculate the enthalpy of formation and Gibbs function of formation for hydrogen peroxide at standard conditions. [15 points] You can use the below table. Thermochemical Properties of Selected Substances at 298K and 1 atm Substance Carbon Hydrogen Nitrogen Oxygen Carbon monoxide Carbon dioxide Water Water Hydrogen peroxide Ammonia Oxygen Hydrogen Formula C(s) H(g) N(g) 0(g) CO(g) CO(g) HO(g) HO(1) HO(g) NH,(g) 0(g) H(g) Molar Mass, M (kg/kmol) 12.01 2.016 28.01 32.00 28.01 44.01 18.02 18.02 34.02 17.03 16.00 1.008 Enthalpy of Formation, T (kJ/kmol) 0 -110,530 -393,520 -241,820 -285,830 -136,310 -46,190 249,170 218,000 Gibbs Function of Formation, gi (kJ/kmol) 0 0 0 0 -137,150 -394,380 -228,590 -237,180 -105,600 -16,590 231,770 203,290 Absolute Entropy, 3 (kJ/kmol K) 5.74 130.57 191.50 205.03 197.54 213.69 188.72 69.95 232.63 192.33 160.95 114.61 Heating Values Higher, HHV (kJ/kg) 32,770 141,780 Lower, LHV 32,770 119,950
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Answes 1 a More information Paused is required for this voltage o...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


