Question
1a. Which of the following types of interactions would this molecule make in a pure solution of this molecule: covalent interactions, ion-ion, ion-dipole, hydrogen bonding
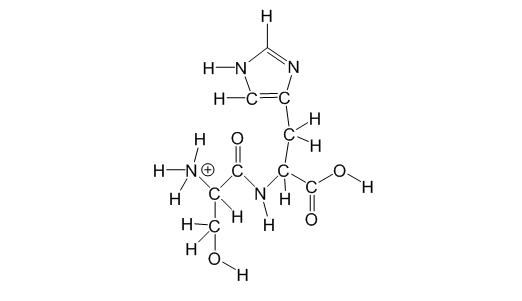
1a. Which of the following types of interactions would this molecule make in a pure solution of this molecule: covalent interactions, ion-ion, ion-dipole, hydrogen bonding interactions and dipole-dipole interactions? This means in the sample there are no other molecules present besides the one shown above. (1 point)
1b. Circle (O) all Hydrogen-bonding acceptor groups and put a triangle () around all Hydrogen-bonding donor groups. (2 points)
1c. Fill in the blanks below about the molecule shown above, in each blank write the correct number which would correctly complete each statement (1 point):
This molecule can make ________ total Hydrogen bonding interactions at most. The strongest interaction this molecule can make with water molecules is a (an) _________ _________________ interaction.
H H-N N -C=C . . H-NO C . C H H-C H HStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


