Answered step by step
Verified Expert Solution
Question
1 Approved Answer
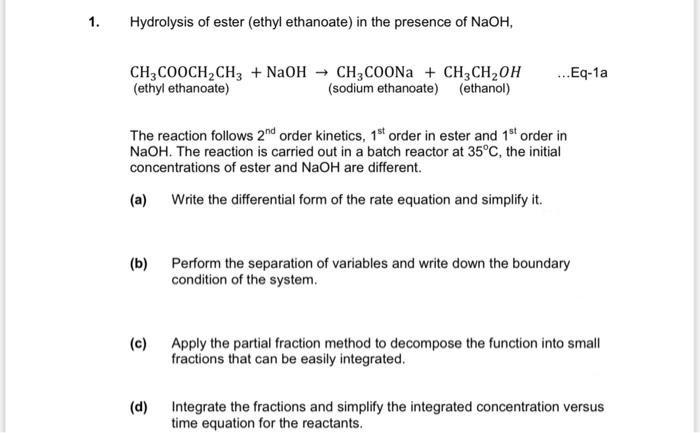
1.Hydrolysis of ester (ethyl ethanoate) in the presence of NaOH, CH3COOCHCH3 + NaOH CH3COONa + CH3CHOH (ethyl ethanoate) (sodium ethanoate) (ethanol) ...Eq-1a The reaction follows
1.Hydrolysis of ester (ethyl ethanoate) in the presence of NaOH, CH3COOCHCH3 + NaOH CH3COONa + CH3CHOH (ethyl ethanoate) (sodium ethanoate) (ethanol) ...Eq-1a
The reaction follows 2nd order kinetics, 1st order in ester and 1st order in NaOH. The reaction is carried out in a batch reactor at 35C, the initial concentrations of ester and NaOH are different.
(a) Write the differential form of the rate equation and simplify it.
(b) Perform the separation of variables and write down the boundary condition of the system.
(c) Apply the partial fraction method to decompose the function into small fractions that can be easily integrated.
(d) Integrate the fractions and simplify the integrated concentration versus time equation for the reactants.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


