Question
1.Prepare a calibration curve by plotting absorbance versus concentration for the four standards on Microsoft Excel. (See below for details.) Draw the best curve through

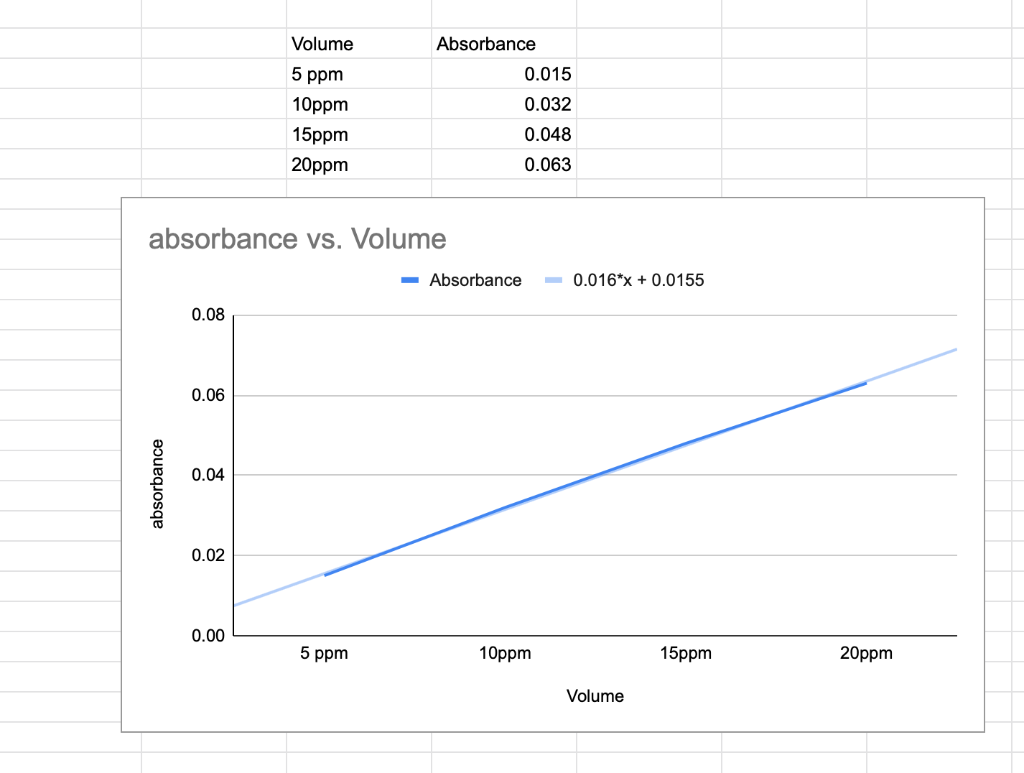
1.Prepare a calibration curve by plotting absorbance versus concentration for the four standards on Microsoft Excel. (See below for details.) Draw the best curve through the data points. > Use your curve to determine the concentration, in ppm, of lead in your sample.
>Calculate the concentration of your saturated solution by multiplying the concentration of your diluted sample by 25000.
2. Convert the concentration of your saturated solution from ppm to mol/L.
(Note:) The density of the solution of Pb(NO3)2 we analyzed is 1.000g/mL.) A sample calculation is shown below. Suppose you find that your saturated solution is 1000 ppm, this is the same as saying that there are 1000 grams of Pb2+ in 1,000,000 grams of solution. This concentration can be converted to molarity as follows. (1000 g Pb2+/106 g solution) * (1.000g solution /mL of solution) * (1000 mL/1 L) * (1 mol / 207.2 g Pb2+) = moles of Pb2+ / L of solution This equation can be simplified to read: (ppm of Pb2+) * (4.826 x 10-6 M/ppm) = Molarity of Pb2+
> Use stoichiometry to determine the concentration, in mol/L, of nitrate in your sample. > Calculate K for the dissolution of lead (II) nitrate in water. > Calculate DG for the dissolution of lead (II) nitrate in water. > Calculate DS for the dissolution of lead (II) nitrate in water.
Please help.
Tod 100 Volume calculate concentration solution" N = 6xloo 5 = 10 x100 15x10o TOS 20x10o = 20 100 absorbance 5ppm 10ppm 15ppm 0.048 Zoppm 0.063 0.037 Find AG 25,000 Pb 2+ + 2NO3 = Pb(NO3) 1 LCPb+Jeq 2([P!2+] ) - [NO3-1 Absorbance ools 0.032 250 ppm = mx + 6 E K = [Pb2+] [NO 1 Volume 5 ppm Absorbance 0.015 0.032 0.048 10ppm 15ppm 20ppm 0.063 absorbance vs. Volume Absorbance -0.016*x + 0.0155 0.08 0.06 0.04 absorbance 0.02 0.00 5 ppm 10ppm 15ppm 20ppm Volume Tod 100 Volume calculate concentration solution" N = 6xloo 5 = 10 x100 15x10o TOS 20x10o = 20 100 absorbance 5ppm 10ppm 15ppm 0.048 Zoppm 0.063 0.037 Find AG 25,000 Pb 2+ + 2NO3 = Pb(NO3) 1 LCPb+Jeq 2([P!2+] ) - [NO3-1 Absorbance ools 0.032 250 ppm = mx + 6 E K = [Pb2+] [NO 1 Volume 5 ppm Absorbance 0.015 0.032 0.048 10ppm 15ppm 20ppm 0.063 absorbance vs. Volume Absorbance -0.016*x + 0.0155 0.08 0.06 0.04 absorbance 0.02 0.00 5 ppm 10ppm 15ppm 20ppm VolumeStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


