Question
1.This experiment was performed and 10.56 grams of distillatewas obtained. The density of the distillate at 25?C wasdetermined to be 0.814 g/mL. Use the below
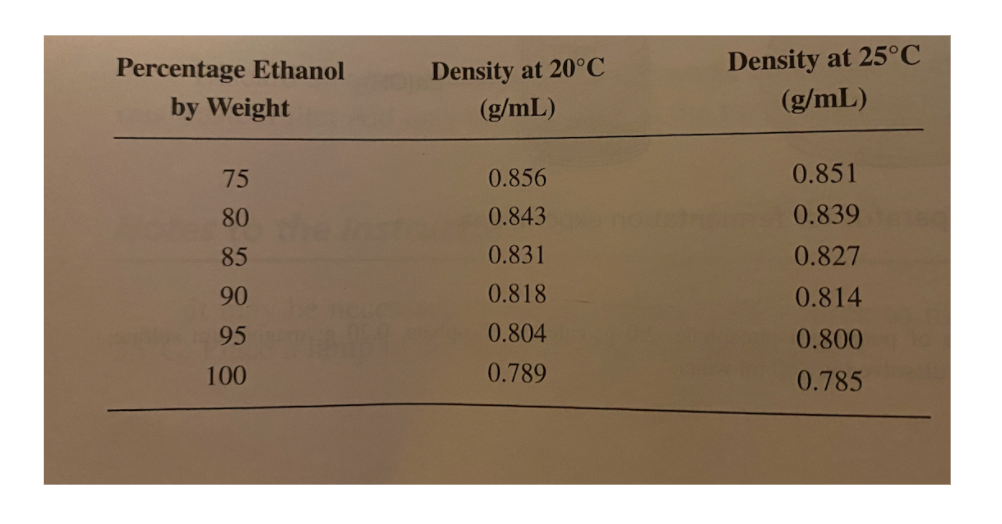
1.This experiment was performed and 10.56 grams of distillatewas obtained. The density of the distillate at 25?C wasdetermined to be 0.814 g/mL. Use the below to calculatethe mass of ethanol, in grams, obtained in the experiment. Showyour calculations.
2. The total volume of fermentation liquid is 200 mL but thelargest distillation flask available for today?s lab will only holda total volume of 50 mL. Instead of performing thedistillation multiple times, we will perform the distillation onlyonce using a representative sample of the fermentation liquid. Ifyou use 40 mL of the fermentation liquid as your representativesample and obtain 1.5 g of ethanol, what mass of ethanol should youuse as your actual yield inyour percent yield calculations?
Percentage Ethanol by Weight 75 80 85 90 95 100 Density at 20C (g/mL) 0.856 0.843 0.831 0.818 0.804 0.789 Density at 25C (g/mL) 0.851 0.839 0.827 0.814 0.800 0.785
Step by Step Solution
3.40 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Answer 1 From the table density 0814 gmL1 at 250C corresponds to 90 ethanol by weig...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


