Answered step by step
Verified Expert Solution
Question
1 Approved Answer
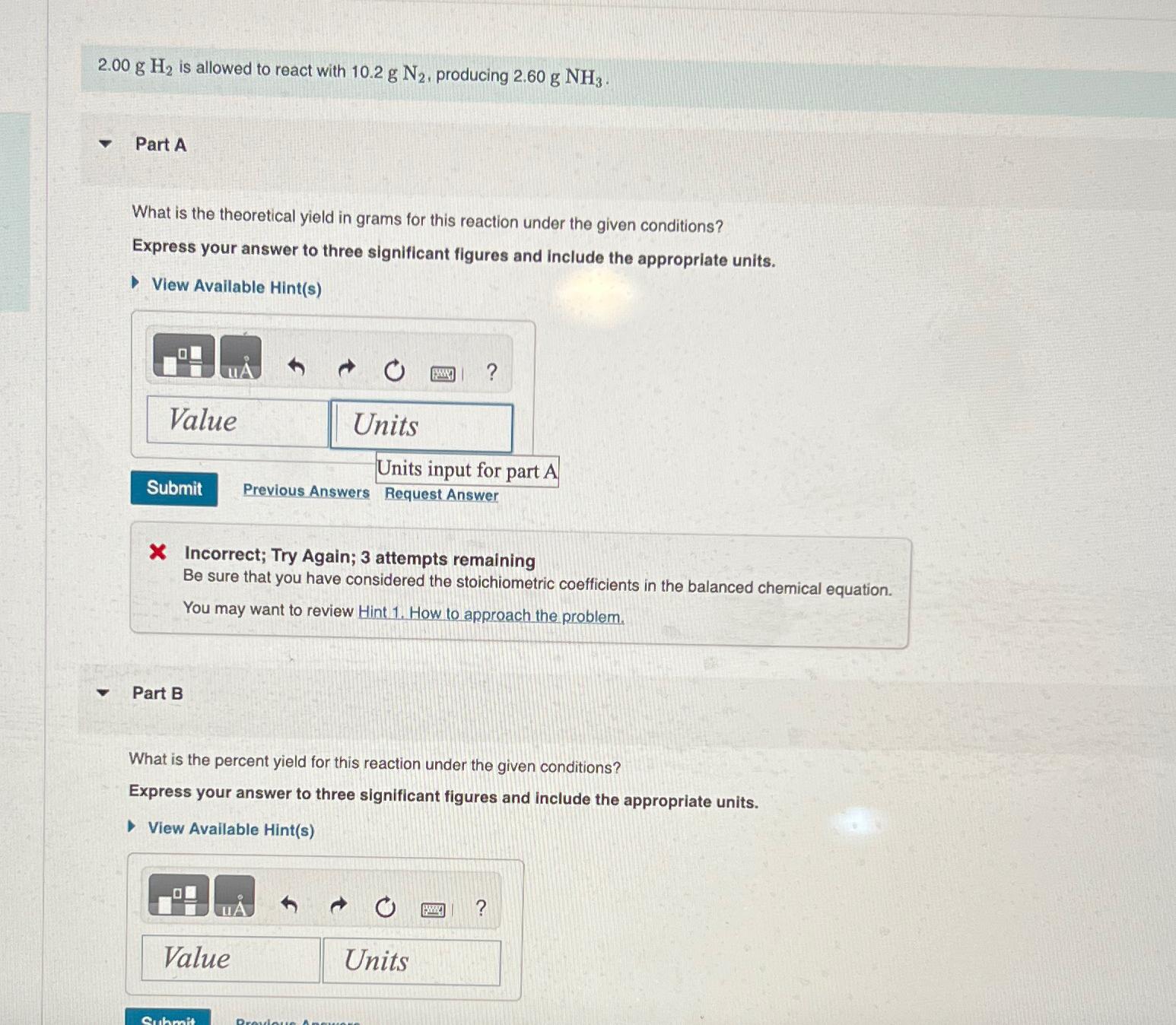
2 . 0 0 g H 2 is allowed to react with 1 0 . 2 g N 2 , producing 2 . 6 0
is allowed to react with producing
Part A
What is the theoretical yield in grams for this reaction under the given conditions?
Express your answer to three significant figures and include the appropriate units.
View Available Hints
Units input for part A
Previous Answers
Request Answer
X Incorrect; Try Again; attempts remaining
Be sure that you have considered the stoichiometric coefficients in the balanced chemical equation.
You may want to review Hint How to approach the problem.
Part B
What is the percent yield for this reaction under the given conditions?
Express your answer to three significant figures and include the appropriate units.
View Available Hints
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


