Answered step by step
Verified Expert Solution
Question
1 Approved Answer
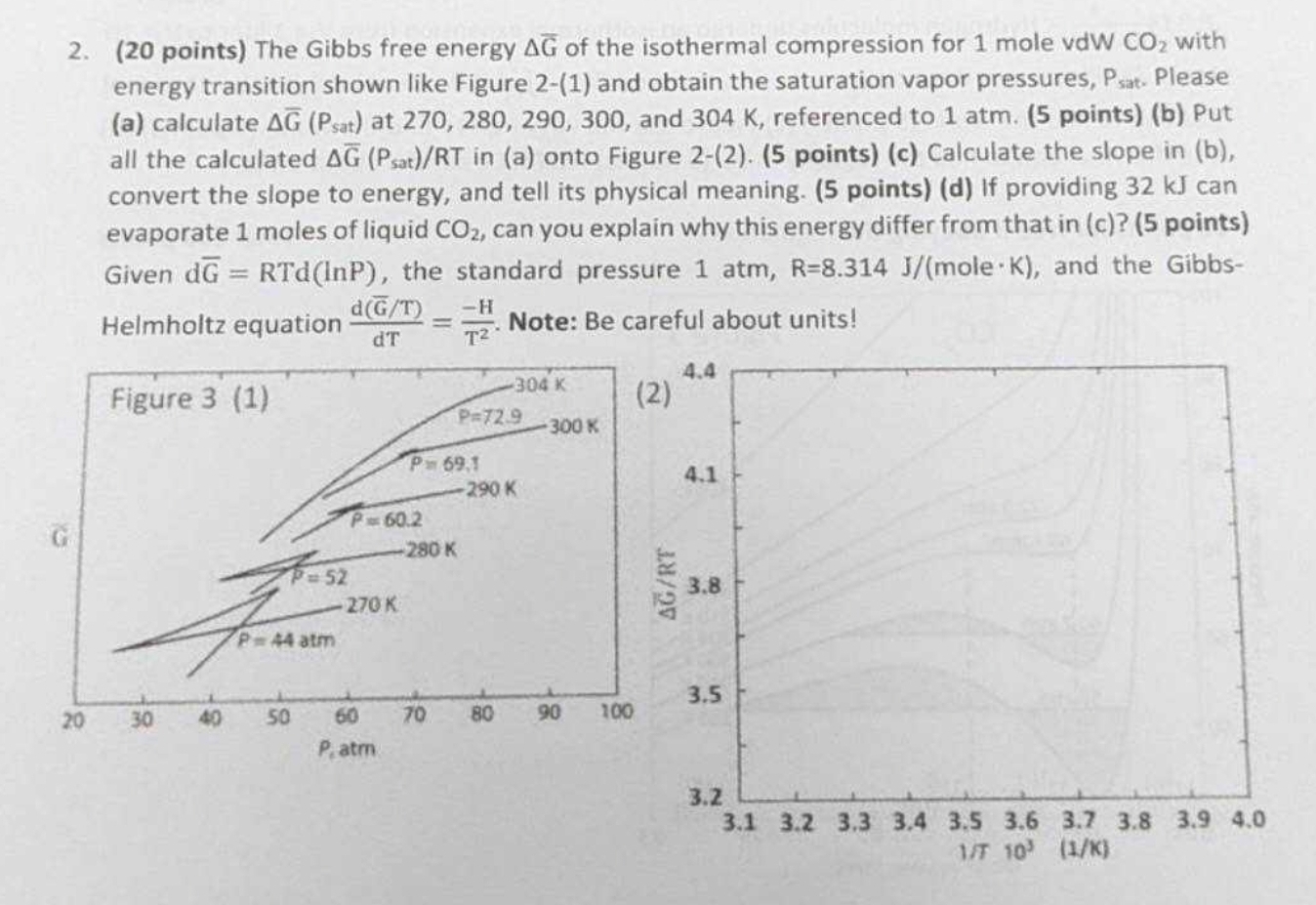
( 2 0 points ) The Gibbs free energy b a r ( G ) of the isothermal compression for 1 mole vdW C O
points The Gibbs free energy of the isothermal compression for mole vdW with energy transition shown like Figure and obtain the saturation vapor pressures, Psat. Please a calculate at and referenced to atm. pointsb Put all the calculated in a onto Figure pointsc Calculate the slope in b convert the slope to energy, and tell its physical meaning. pointsd If providing can evaporate moles of liquid can you explain why this energy differ from that in c points Given the standard pressure mole and the GibbsHelmholtz equation Note: Be careful about units!
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


