Answered step by step
Verified Expert Solution
Question
1 Approved Answer
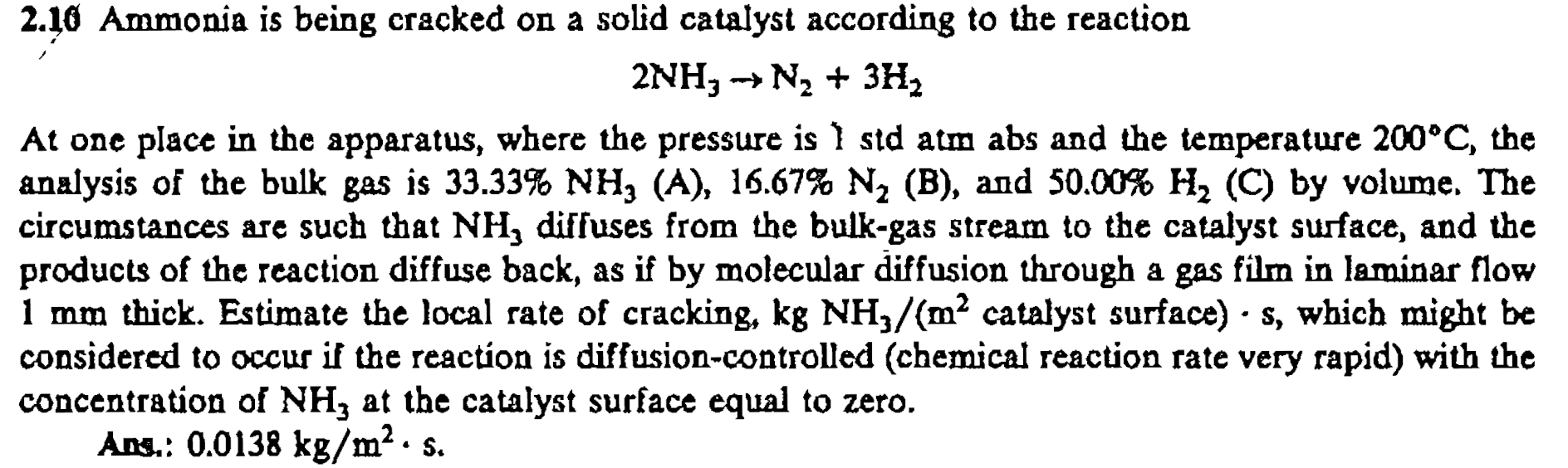
2 . 1 0 Ammonia is being cracked on a solid catalyst according to the reaction 2 N H 3 N 2 + 3 H
Ammonia is being cracked on a solid catalyst according to the reaction
At one place in the apparatus, where the pressure is atm abs and the temperature the
analysis of the bulk gas is B and C by volume. The
circumstances are such that diffuses from the bulkgas stream to the catalyst surface, and the
products of the reaction diffuse back, as if by molecular diffusion through a gas film in laminar flow
thick. Estimate the local rate of cracking,
considered to occur if the reaction is diffusioncontrolled chemical reaction rate very rapid with the
concentration of at the catalyst surface equal to zero.
Ans.:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


