Answered step by step
Verified Expert Solution
Question
1 Approved Answer
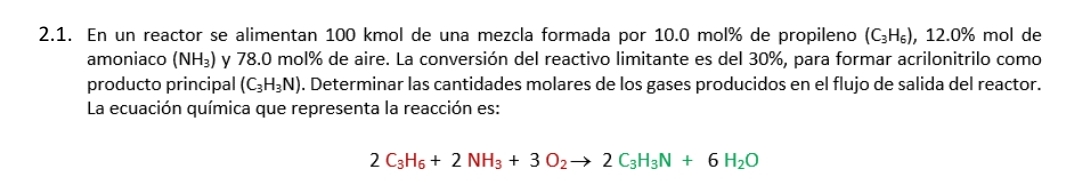
2 . 1 . En un reactor se alimentan 1 0 0 kmol de una mezcla formada por 1 0 . 0 mol % de
En un reactor se alimentan kmol de una mezcla formada por mol de propileno mol de amoniaco y mol de aire. La conversin del reactivo limitante es del para formar acrilonitrilo como producto principal Determinar las cantidades molares de los gases producidos en el flujo de salida del reactor. La ecuacin qumica que representa la reaccin es:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


