Question
A sample of commercial perchloric acid is 85.0% HCIO4 by mass; its density is 1.664 g/mL. How many milliliters of this concentrated HCIO4 would
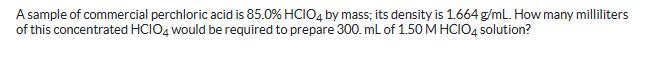
A sample of commercial perchloric acid is 85.0% HCIO4 by mass; its density is 1.664 g/mL. How many milliliters of this concentrated HCIO4 would be required to prepare 300. mL of 1.50 M HCIO4 solution?
Step by Step Solution
3.37 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Financial Reporting Financial Statement Analysis And Valuation A Strategic Perspective
Authors: James M. Wahlen, Stephen P. Baginski, Mark Bradshaw
9th Edition
1337614689, 1337614688, 9781337668262, 978-1337614689
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



