Answered step by step
Verified Expert Solution
Question
1 Approved Answer
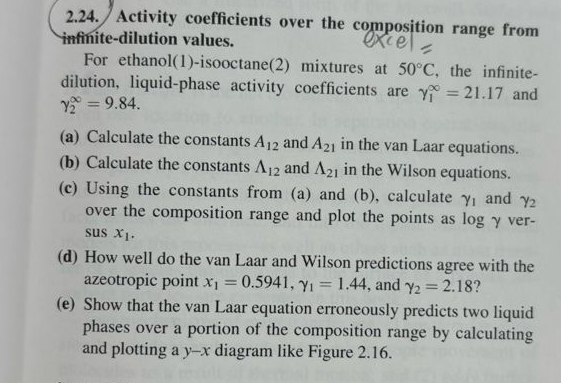
2 . 2 4 . Activity coefficients over the composition range from infinite - dilution values. For ethanol ( 1 ) - isooctane ( 2
Activity coefficients over the composition range from infinitedilution values.
For ethanolisooctane mixtures at the infinitedilution, liquidphase activity coefficients are and
a Calculate the constants and in the van Laar equations.
b Calculate the constants and in the Wilson equations.
c Using the constants from a and b calculate and over the composition range and plot the points as versus
d How well do the van Laar and Wilson predictions agree with the azeotropic point and
e Show that the van Laar equation erroneously predicts two liquid phases over a portion of the composition range by calculating and plotting a diagram like Figure
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


