Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. (25 pts) Dry air is defined as air with no water vapor, and the molecular weight of air, Mair = 28.97 kg/kmol, is
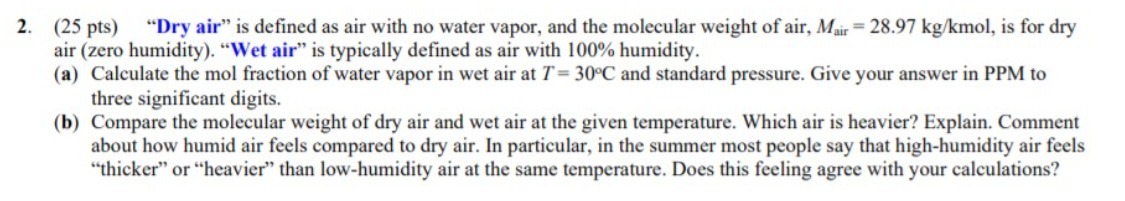
2. (25 pts) "Dry air" is defined as air with no water vapor, and the molecular weight of air, Mair = 28.97 kg/kmol, is for dry air (zero humidity). "Wet air" is typically defined as air with 100% humidity. (a) Calculate the mol fraction of water vapor in wet air at T=30C and standard pressure. Give your answer in PPM to three significant digits. (b) Compare the molecular weight of dry air and wet air at the given temperature. Which air is heavier? Explain. Comment about how humid air feels compared to dry air. In particular, in the summer most people say that high-humidity air feels "thicker" or "heavier" than low-humidity air at the same temperature. Does this feeling agree with your calculations?
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a To calculate the mole fraction of water vapor in wet air we need to know the partial pressure of water vapor at the given temperature At standard pr...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


