Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2, 3, 14 please answer all parts of the question Visible light has wavelengths from 400 to 700 nm, whereas the wavelength region for X-ray
2, 3, 14 please answer all parts of the question 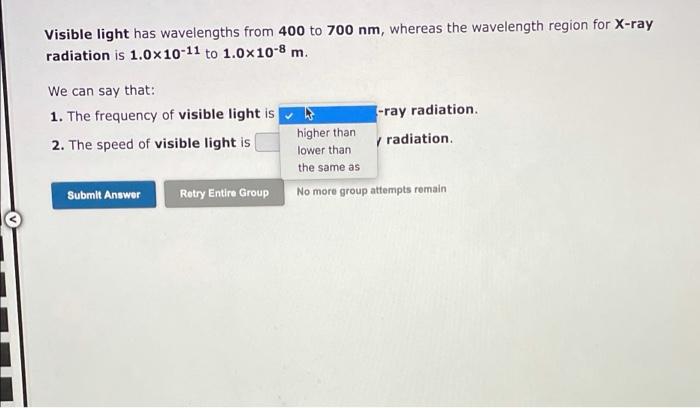
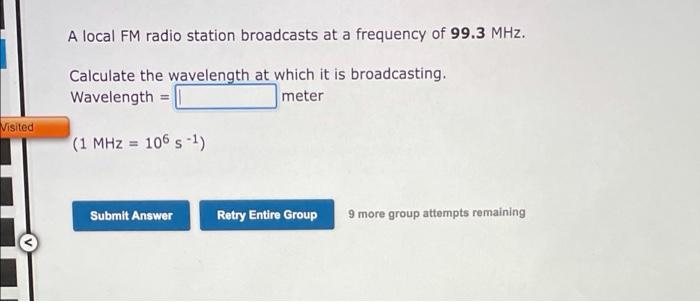
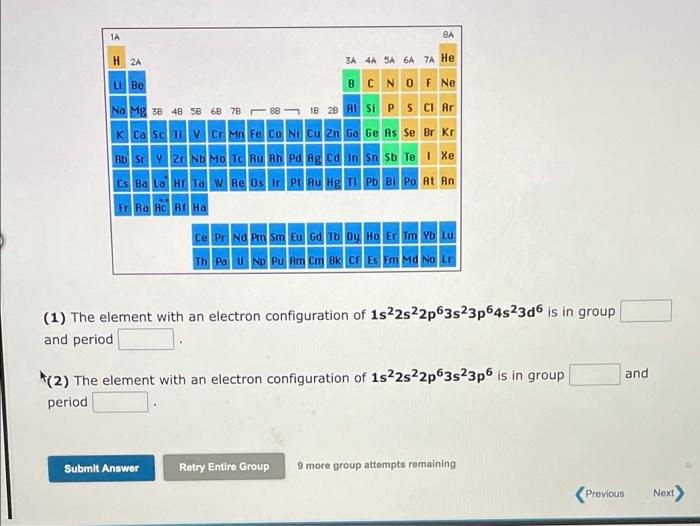
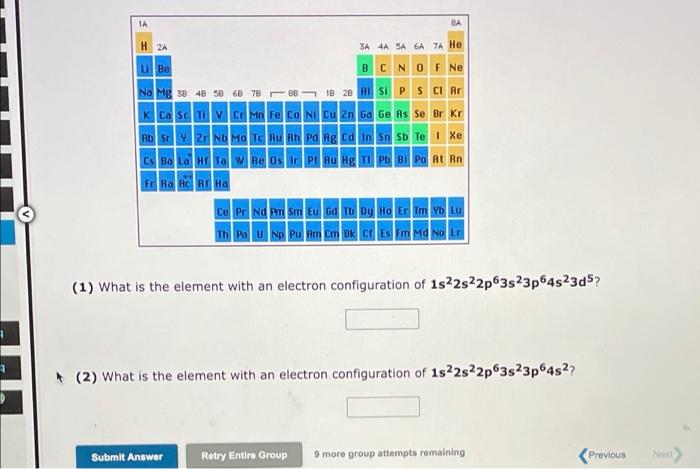
Visible light has wavelengths from 400 to 700 nm, whereas the wavelength region for X-ray radiation is 1.0x10-11 to 1.0x10-8 m. We can say that: 1. The frequency of visible light is 2. The speed of visible light is -ray radiation higher than radiation lower than the same as No more group attempts romain Submit Answer Retry Entire Group A local FM radio station broadcasts at a frequency of 99.3 MHz. Calculate the wavelength at which it is broadcasting, Wavelength = meter Visited (1 MHz = 106 s-1) Submit Answer Retry Entire Group 9 more group attempts remaining 1A H 2A 3A 4A SA 6A 7A He LiBe B C N O F Ne Na Mg x 48 58 68 78 08 10 28 Al Si P S Cl Ar K Ca Sc Ti V CI Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rosy | 2 Nh Ma - Tu Pa lg Ed n on sb Te | Xe Cs Ba LOHI TA W Re Os Ir PL RU Hg Tl Pb Bi Po At Rn FRA AO RI Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk CI Es Fm Md No Lr (1) The element with an electron configuration of 1s22s22p63s23p64s23d6 is in group and period and (2) The element with an electron configuration of 1s22s22p63s23p is in group period Submit Answer Retry Entire Group 9 more group attempts remaining Previous Next) 1A BA H 2A 3A 4A 5A6A7A He u Be O BCN O F Ne Na Mg x 48 5 6 7820 B 28 BS P S Cl Ar K Ca Sc CI Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr HD | P - Nh Ma Tc nu nh ng ng d n sn su Te | Xe Cs Ba Lata Re Os LPL RU Hg Ph Bi Po At Rn FRA RC RE HA Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb LU Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr (1) What is the element with an electron configuration of 1s22s22p63s23p6452305? 1 2 (2) What is the element with an electron configuration of 1s22s22p63s23p64527 Submit Answer Retry Entire Group 9 more group attempts remaining Previous 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


