Answered step by step
Verified Expert Solution
Question
1 Approved Answer
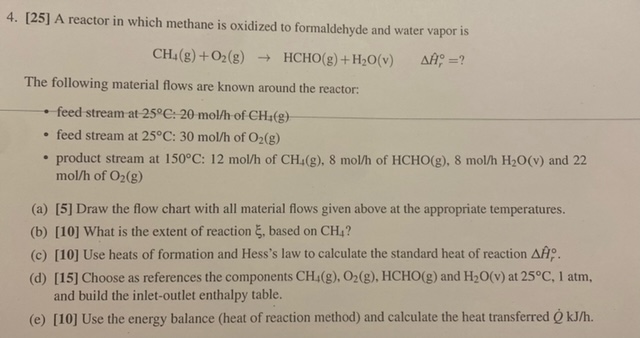
[ 2 5 ] A reactor in which methane is oxidized to formaldehyde and water vapor is C H 4 ( g ) + O
A reactor in which methane is oxidized to formaldehyde and water vapor is
HCHO
The following material flows are known around the reactor:
feed stream at :
feed stream at : of
product stream at : of of HCHO and
of
a Draw the flow chart with all material flows given above at the appropriate temperatures.
b What is the extent of reaction based on
c Use heats of formation and Hess's law to calculate the standard heat of reaction
d Choose as references the components HCHO and at atm,
and build the inletoutlet enthalpy table.
e Use the energy balance heat of reaction method and calculate the heat transferred
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


