Answered step by step
Verified Expert Solution
Question
1 Approved Answer
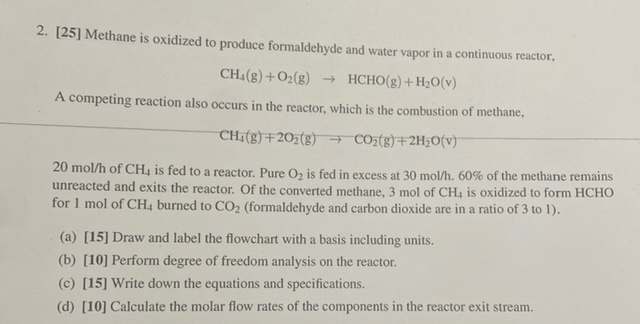
[ 2 5 ] Methane is oxidized to produce formaldehyde and water vapor in a continuous reactor, C H 4 ( g ) + O
Methane is oxidized to produce formaldehyde and water vapor in a continuous reactor,
HCHO
A competing reaction also occurs in the reactor, which is the combustion of methane,
of is fed to a reactor. Pure is fed in excess at of the methane remains
unreacted and exits the reactor. Of the converted methane, is oxidized to form HCHO
for burned to formaldehyde and carbon dioxide are in a ratio of to
a Draw and label the flowchart with a basis including units.
b Perform degree of freedom analysis on the reactor.
c Write down the equations and specifications.
d Calculate the molar flow rates of the components in the reactor exit stream.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


