Answered step by step
Verified Expert Solution
Question
1 Approved Answer
( 2 5 % ) Methanol can be made by catalytic oxidation of methane using the stoichiometric amount of O 2 as shown in the
Methanol can be made by catalytic oxidation of methane using the stoichiometric amount of as shown in the reaction below. A conversion of of the methane was obtained in the reactor. Determine the quantity of heat required to be added or removed from the reactor.
Basis of calculation: mole of methane fed.
Given: the Constant heat capacity, of and are and respectively You can use or table for calculation.
Feed temperature: for Methane; for Oxygen
Product temperature:
Standard Heat of reaction is
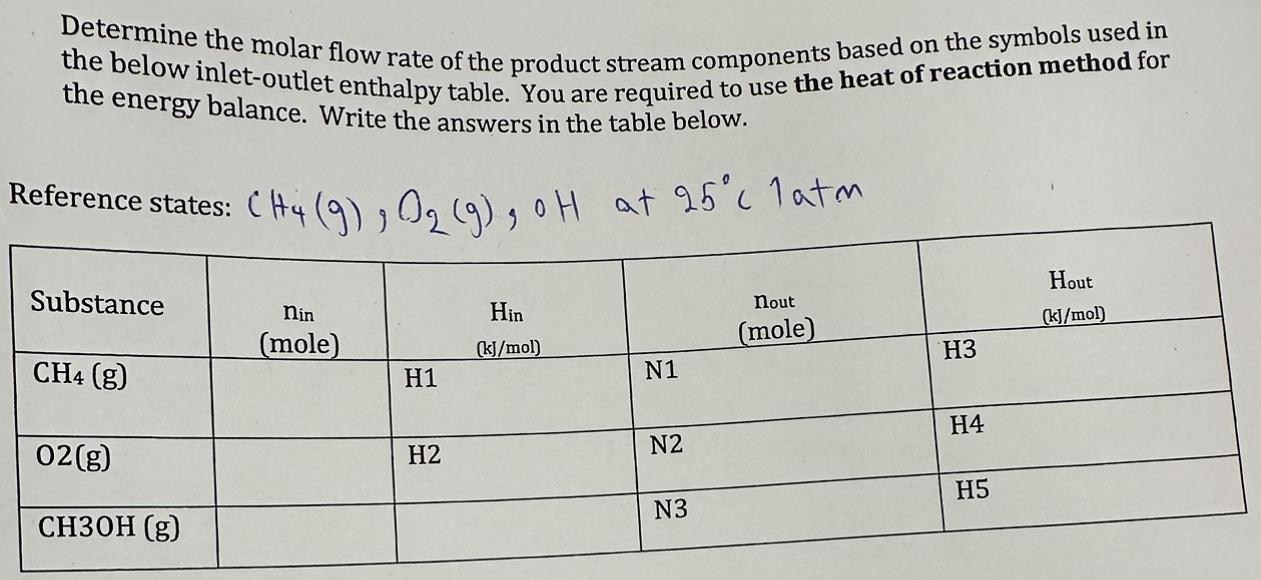
Determine the molar flow rate of the product stream components based on the symbols used in the below inletoutlet enthalpy table. You are required to use the heat of reaction method for the energy balance. Write the answers in the table below.
Reference states: at latm
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


