Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2) 5.00-mL aliquots of a sample containing unknown amounts of phenobarbital were measured into 50.00- mL volumetric flasks and made basic with KOH. The following
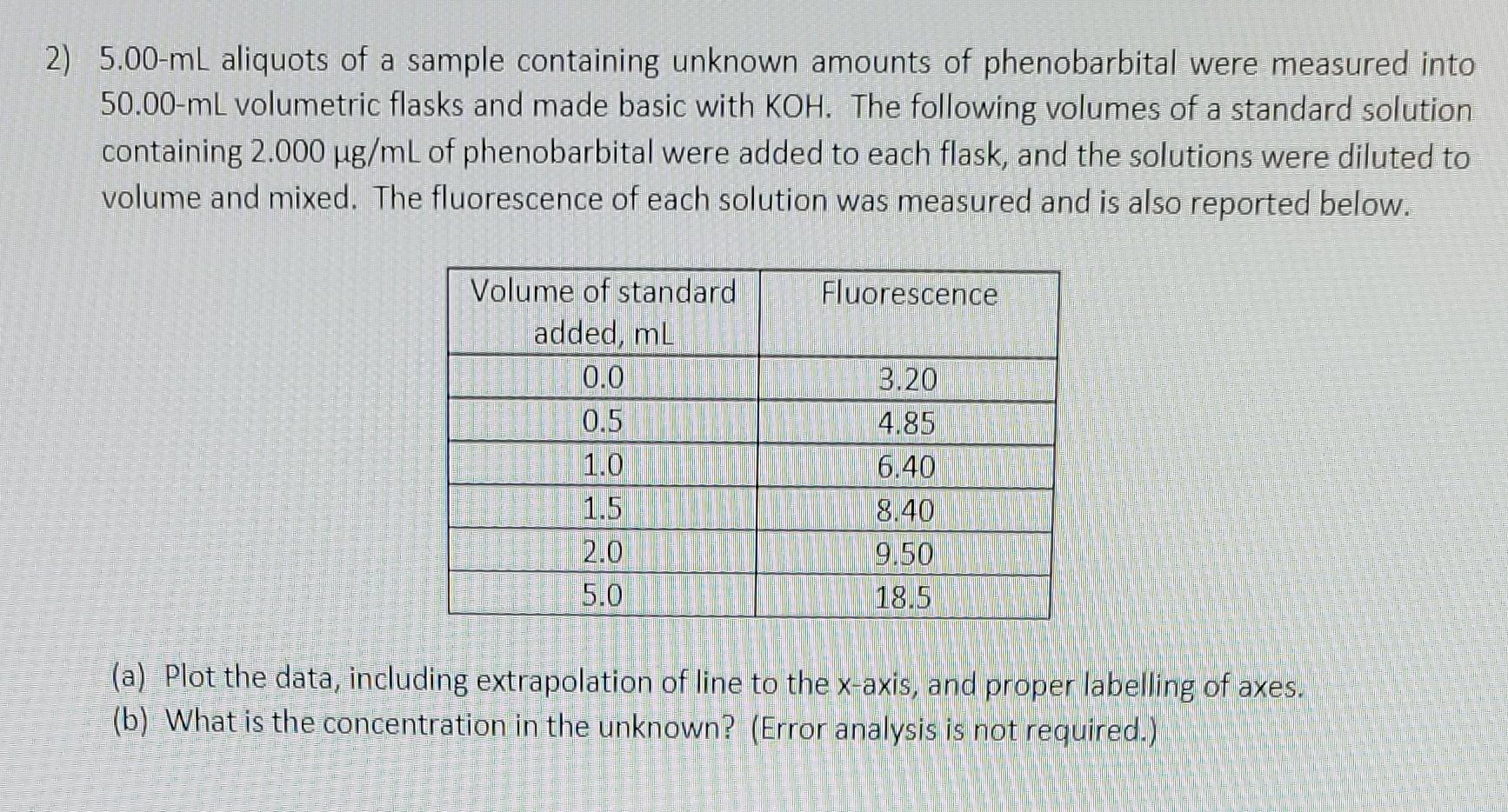
2) 5.00-mL aliquots of a sample containing unknown amounts of phenobarbital were measured into 50.00- mL volumetric flasks and made basic with KOH. The following volumes of a standard solution containing 2.000g/mL of phenobarbital were added to each flask, and the solutions were diluted to volume and mixed. The fluorescence of each solution was measured and is also reported below. (a) Plot the data, including extrapolation of line to the x-axis, and proper labelling of axes. (b) What is the concentration in the unknown? (Error analysis is not required.)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


