Answered step by step
Verified Expert Solution
Question
1 Approved Answer
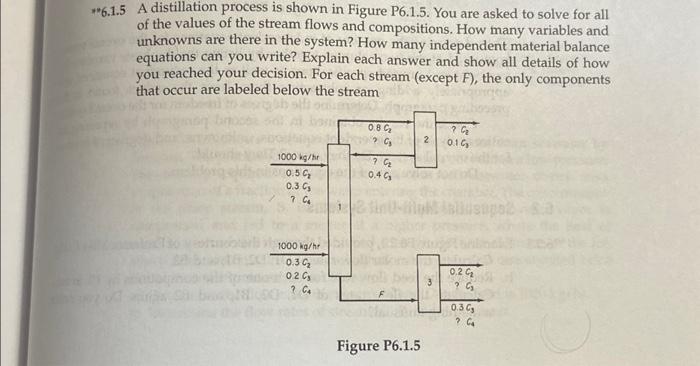
2 **6.1.5 A distillation process is shown in Figure P6.1.5. You are asked to solve for all of the values of the stream flows and
2 **6.1.5 A distillation process is shown in Figure P6.1.5. You are asked to solve for all of the values of the stream flows and compositions. How many variables and unknowns are there in the system? How many independent material balance equations can you write? Explain each answer and show all details of how you reached your decision. For each stream (except F), the only components that occur are labeled below the stream 1000 kg/hr 20.5 C 0.3 C3 ? Ca 1000 kg/hr 0.3 C 0.2 C3 ? C4 0.8 C ? C3 ? C 0.4 03 Figure P6.1.5 2 3 ? C 0.1 C3 0.2 C ? C3 0.3 C3 ? C4
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


