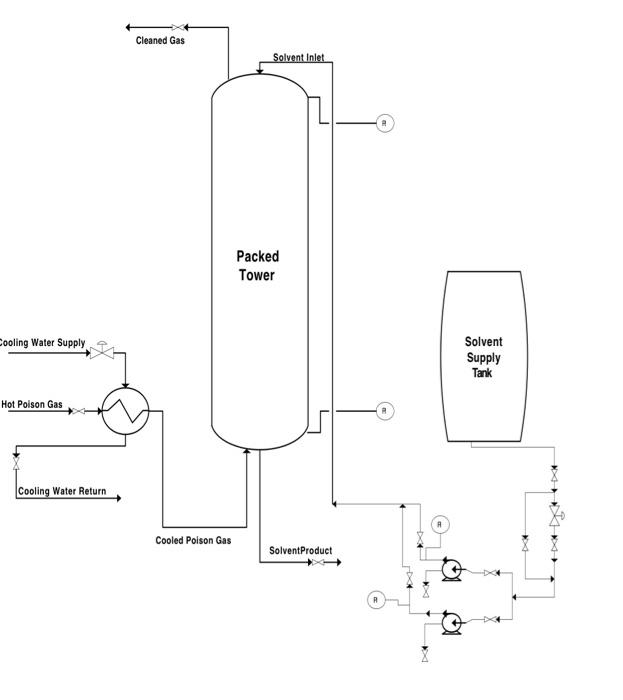
2. A countercurrent wet-film packed tower is used to absorb CO2 from a poisonous gas stream into a liquid solvent phase. The liquid solvent, NaOH, enters at the top of the column and is contacted by the poisonous gas stream entering at the bottom of the column. Upon contact, the CO2 in the gas phase is absorbed into the liquid solvent phase. The poisonous gas must be cooled from 400C to 80C before entering the tower to ensure that the solvent does not vaporize in the tower, resulting in a possible explosion due to a sharp increase in column pressure. A heat exchanger adjacent to the column provides this necessary cooling. The resulting liquid mixture, upon absorption of CO2 into the liquid phase is a valuable product (Na2CO3) of the process and is reused downstream. Our main objectives are to control the concentration of CO2 in the gas stream exiting the top of the column after absorption and maintain the temperature of the poisonous gas entering the column at a safe temperature (i.e., below 80C ). A. Develop control loops to achieve the two specified objectives. Your control of the gas concentration should be dependent upon the solvent flow entering the column. Be mindful that there should always be an adequate level of liquid in the solvent tank. "PI" represents pressure indicators but other measurements/sensors can be added as needed. B. Assume a failure of your control loop for maintaining the gas temperature at a safe value (i.e., it gets too hot and can now vaporize the solvent in the column). Suggest a method to prevent tower explosion due to high column pressure assuming a temperature value of the entering hot gas cannot be obtained. Solvent Inlet, Packed Tower Solvent Supply Tank Cooled Poison Gas SolventProduct 2. A countercurrent wet-film packed tower is used to absorb CO2 from a poisonous gas stream into a liquid solvent phase. The liquid solvent, NaOH, enters at the top of the column and is contacted by the poisonous gas stream entering at the bottom of the column. Upon contact, the CO2 in the gas phase is absorbed into the liquid solvent phase. The poisonous gas must be cooled from 400C to 80C before entering the tower to ensure that the solvent does not vaporize in the tower, resulting in a possible explosion due to a sharp increase in column pressure. A heat exchanger adjacent to the column provides this necessary cooling. The resulting liquid mixture, upon absorption of CO2 into the liquid phase is a valuable product (Na2CO3) of the process and is reused downstream. Our main objectives are to control the concentration of CO2 in the gas stream exiting the top of the column after absorption and maintain the temperature of the poisonous gas entering the column at a safe temperature (i.e., below 80C ). A. Develop control loops to achieve the two specified objectives. Your control of the gas concentration should be dependent upon the solvent flow entering the column. Be mindful that there should always be an adequate level of liquid in the solvent tank. "PI" represents pressure indicators but other measurements/sensors can be added as needed. B. Assume a failure of your control loop for maintaining the gas temperature at a safe value (i.e., it gets too hot and can now vaporize the solvent in the column). Suggest a method to prevent tower explosion due to high column pressure assuming a temperature value of the entering hot gas cannot be obtained. Solvent Inlet, Packed Tower Solvent Supply Tank Cooled Poison Gas SolventProduct