Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2 A frictionless piston/cylinder assembly A shown in the figure is connected by a thin pipeline with a valve to a rigid tank B. The
2 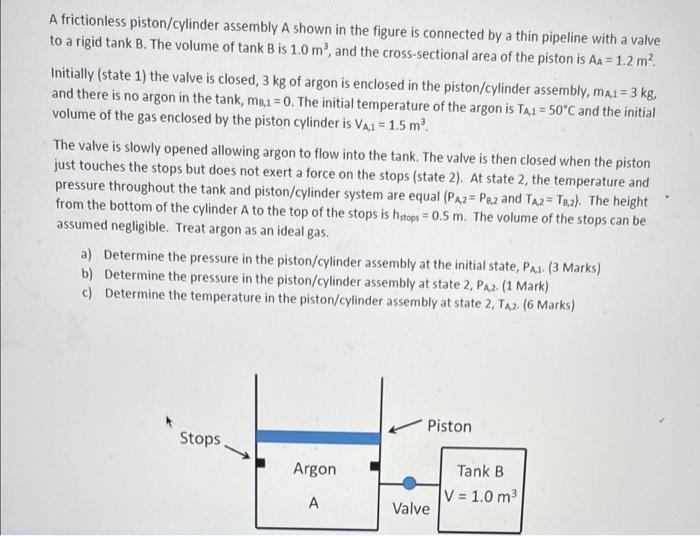
A frictionless piston/cylinder assembly A shown in the figure is connected by a thin pipeline with a valve to a rigid tank B. The volume of tank B is 1.0 m, and the cross-sectional area of the piston is AA = 1.2 m? Initially (state 1) the valve is closed, 3 kg of argon is enclosed in the piston/cylinder assembly, mai = 3 kg, and there is no argon in the tank, m8,1 = 0. The initial temperature of the argon is TA1 = 50C and the initial volume of the gas enclosed by the piston cylinder is V2,1 = 1.5 m The valve is slowly opened allowing argon to flow into the tank. The valve is then closed when the piston just touches the stops but does not exert a force on the stops (state 2). At state 2, the temperature and pressure throughout the tank and piston/cylinder system are equal (PA2=Paz and TA2 = T3,2). The height from the bottom of the cylinder A to the top of the stops is hstops = 0.5 m. The volume of the stops can be assumed negligible. Treat argon as an ideal gas. a) Determine the pressure in the piston/cylinder assembly at the initial state, Pal (3 Marks) b) Determine the pressure in the piston/cylinder assembly at state 2, Paz (1 Mark) c) Determine the temperature in the piston/cylinder assembly at state 2, TA2 (6 Marks) Piston Stops Argon Tank B V = 1.0 m2 Valve A 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


