Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A gas has composition as given below. Determine a value of z-factor for this gas at reservoir conditions of 5720 psig and 268F. Component
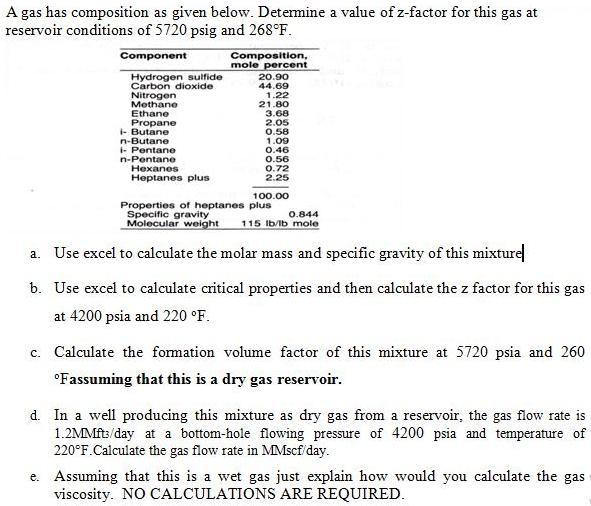
A gas has composition as given below. Determine a value of z-factor for this gas at reservoir conditions of 5720 psig and 268F. Component Hydrogen sulfide Carbon dioxide Nitrogen Methane Ethane Propane i-Butane n-Butane i-Pentane n-Pentane Hexanes Heptanes plus Composition, mole percent 20.90 44.69 1.22 21.80 3.68 2.05 0.58 1.09 0.46 0.56 0.72 2.25 100.00 Properties of heptanes plus Specific gravity 0.844 Molecular weight 115 lb/lb mole a. Use excel to calculate the molar mass and specific gravity of this mixture b. Use excel to calculate critical properties and then calculate the z factor for this gas at 4200 psia and 220 F. c. Calculate the formation volume factor of this mixture at 5720 psia and 260 Fassuming that this is a dry gas reservoir. d. In a well producing this mixture as dry gas from a reservoir, the gas flow rate is 1.2MMft/day at a bottom-hole flowing pressure of 4200 psia and temperature of 220F.Calculate the gas flow rate in MMscf/day. e. Assuming that this is a wet gas just explain how would you calculate the gas viscosity. NO CALCULATIONS ARE REQUIRED.
Step by Step Solution
★★★★★
3.63 Rating (179 Votes )
There are 3 Steps involved in it
Step: 1
Step 1 The pressure of the wet gas P 5720 psi The temperature of the wet gas T 268F 72767R The given ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


