Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Alkaloid G, C8H13NO, is a tertiary base. It has IR absorption at 3500 cm and H NMR signals at 8 3.0 (5H, m), 3.8
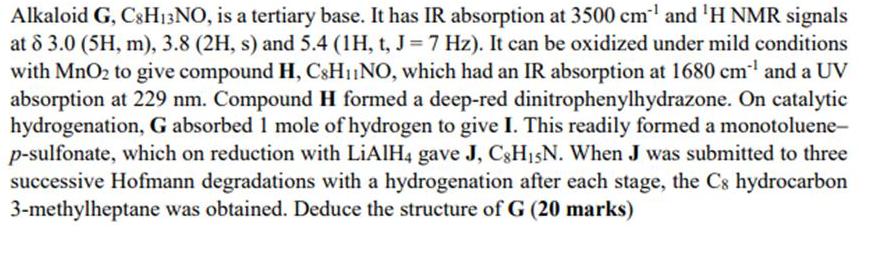
Alkaloid G, C8H13NO, is a tertiary base. It has IR absorption at 3500 cm and H NMR signals at 8 3.0 (5H, m), 3.8 (2H, s) and 5.4 (1H, t, J = 7 Hz). It can be oxidized under mild conditions with MnO to give compound H, C8H1NO, which had an IR absorption at 1680 cm and a UV absorption at 229 nm. Compound H formed a deep-red dinitrophenylhydrazone. On catalytic hydrogenation, G absorbed 1 mole of hydrogen to give I. This readily formed a monotoluene- p-sulfonate, which on reduction with LiAlH4 gave J, C8H15N. When J was submitted to three successive Hofmann degradations with a hydrogenation after each stage, the C8 hydrocarbon 3-methylheptane was obtained. Deduce the structure of G (20 marks)
Step by Step Solution
★★★★★
3.48 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
To deduce the structure of alkaloid G we will use the information given about its spectroscopic properties and the reactions it undergoes IR absorptio...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


