Question
(c) A volatile plant product M, C8H140, has strong IR absorption at 1717 cm. It possess H NMR signals at 8H 1.65 (3H, s),
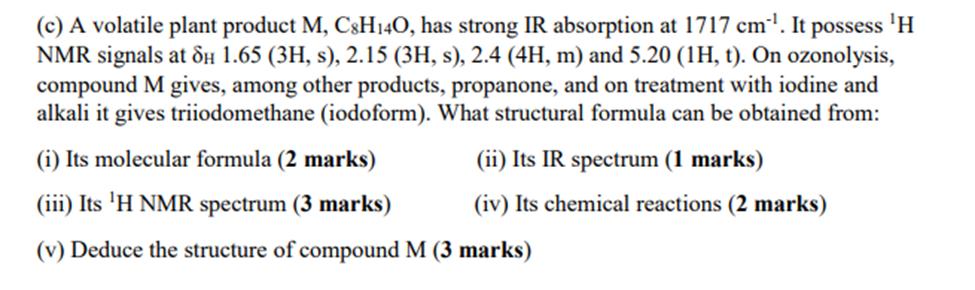
(c) A volatile plant product M, C8H140, has strong IR absorption at 1717 cm. It possess H NMR signals at 8H 1.65 (3H, s), 2.15 (3H, s), 2.4 (4H, m) and 5.20 (1H, t). On ozonolysis, compound M gives, among other products, propanone, and on treatment with iodine and alkali it gives triiodomethane (iodoform). What structural formula can be obtained from: (ii) Its IR spectrum (1 marks) (iv) Its chemical reactions (2 marks) (i) Its molecular formula (2 marks) (iii) Its 'H NMR spectrum (3 marks) (v) Deduce the structure of compound M (3 marks)
Step by Step Solution
3.49 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1
i The molecular formula of compound M is C8H14O ii The strong IR absorption at 1717 cm1 indicates th...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Graham Solomons, Craig Fryhle, Scott Snyder
11th edition
1118133579, 978-1118133576
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



