Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2 and 5 are correct but I dont know which one is the incorrect one Select one incorrect statement. When the phase diagram of a
2 and 5 are correct but I dont know which one is the incorrect one 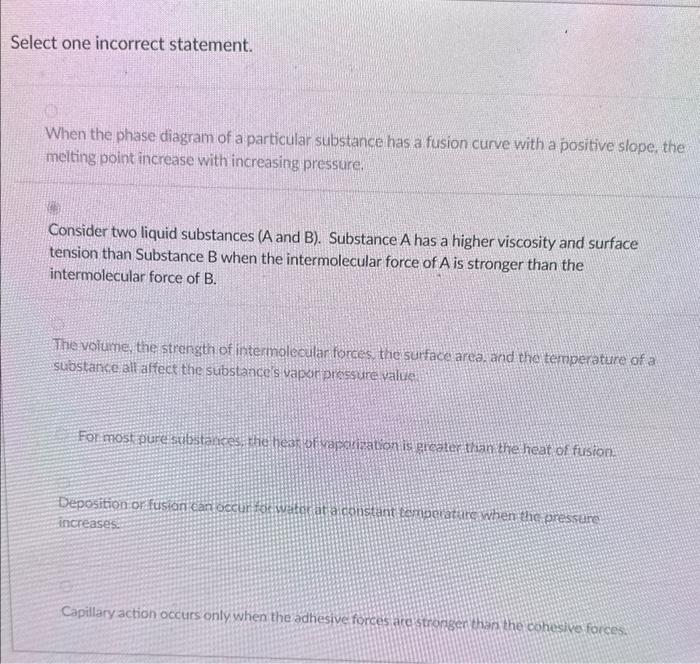
Select one incorrect statement. When the phase diagram of a particular substance has a fusion curve with a positive slope, the melting point increase with increasing pressure. Consider two liquid substances (A and B). Substance A has a higher viscosity and surface tension than Substance B when the intermolecular force of A is stronger than the intermolecular force of B. The voiume the strength of intermalecular forces, the surface area, and the temperature of a substanceiall affect the substance s vapor pirsesure value Capillary action occurs only when the adhesive forces arcistronser than he cohesive forces 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


