Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. Assume that you weigh 0.4000 g of sample that is pure NaCl and subject it to the procedure described in this lab. How
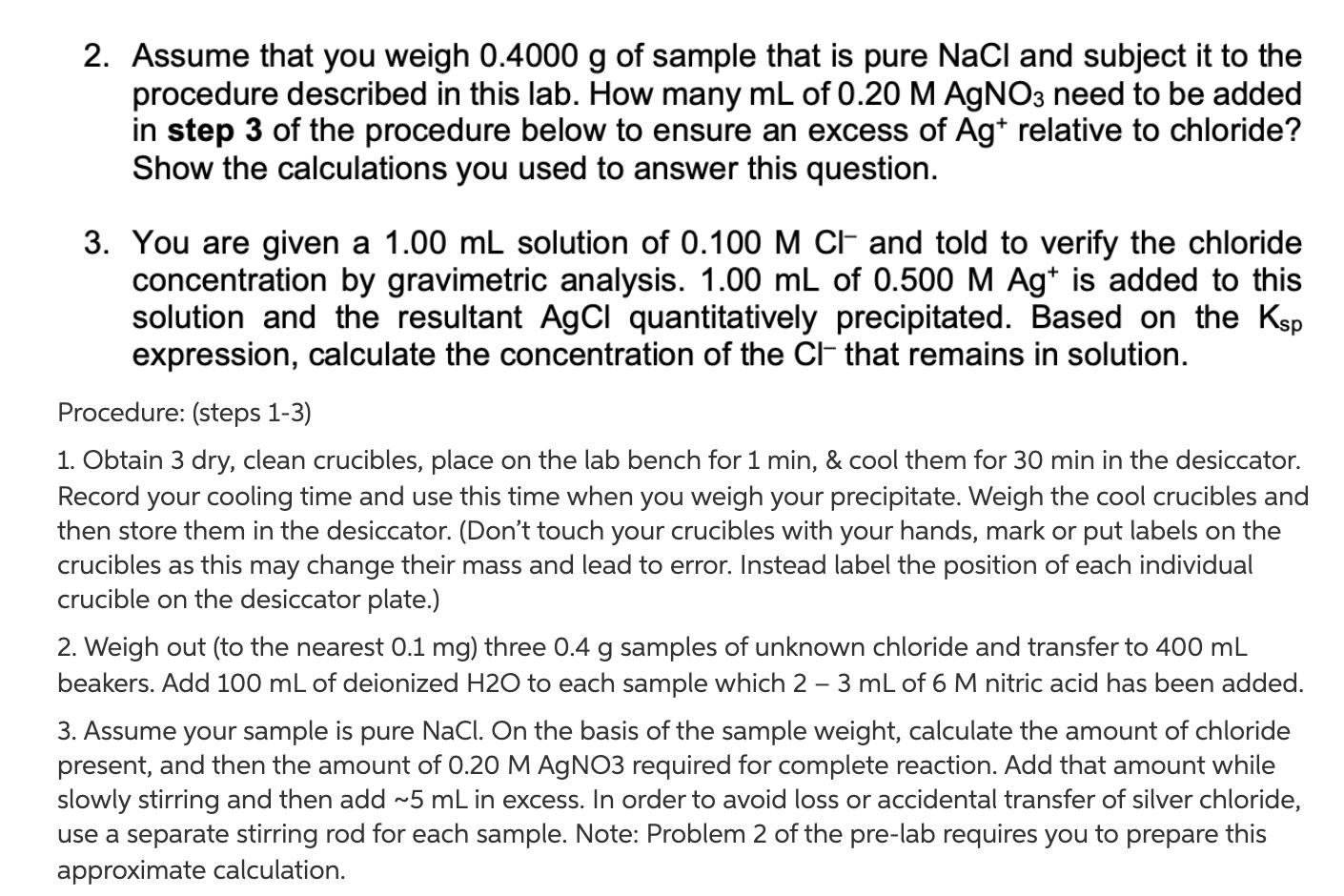
2. Assume that you weigh 0.4000 g of sample that is pure NaCl and subject it to the procedure described in this lab. How many mL of 0.20 M AgNO3 need to be added in step 3 of the procedure below to ensure an excess of Ag+ relative to chloride? Show the calculations you used to answer this question. 3. You are given a 1.00 mL solution of 0.100 M CI- and told to verify the chloride concentration by gravimetric analysis. 1.00 mL of 0.500 M Ag+ is added to this solution and the resultant AgCl quantitatively precipitated. Based on the Ksp expression, calculate the concentration of the Cl- that remains in solution. Procedure: (steps 1-3) 1. Obtain 3 dry, clean crucibles, place on the lab bench for 1 min, & cool them for 30 min in the desiccator. Record your cooling time and use this time when you weigh your precipitate. Weigh the cool crucibles and then store them in the desiccator. (Don't touch your crucibles with your hands, mark or put labels on the crucibles as this may change their mass and lead to error. Instead label the position of each individual crucible on the desiccator plate.) 2. Weigh out (to the nearest 0.1 mg) three 0.4 g samples of unknown chloride and transfer to 400 mL beakers. Add 100 mL of deionized H2O to each sample which 2 - 3 mL of 6 M nitric acid has been added. 3. Assume your sample is pure NaCl. On the basis of the sample weight, calculate the amount of chloride present, and then the amount of 0.20 M AgNO3 required for complete reaction. Add that amount while slowly stirring and then add ~5 mL in excess. In order to avoid loss or accidental transfer of silver chloride, use a separate stirring rod for each sample. Note: Problem 2 of the pre-lab requires you to prepare this approximate calculation.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


