Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2 attempts left Check my work Be sure to answer all parts. A 5.292-g sample of a mixture of cyclohexane (C H12) and naphthalene (C10Hz)
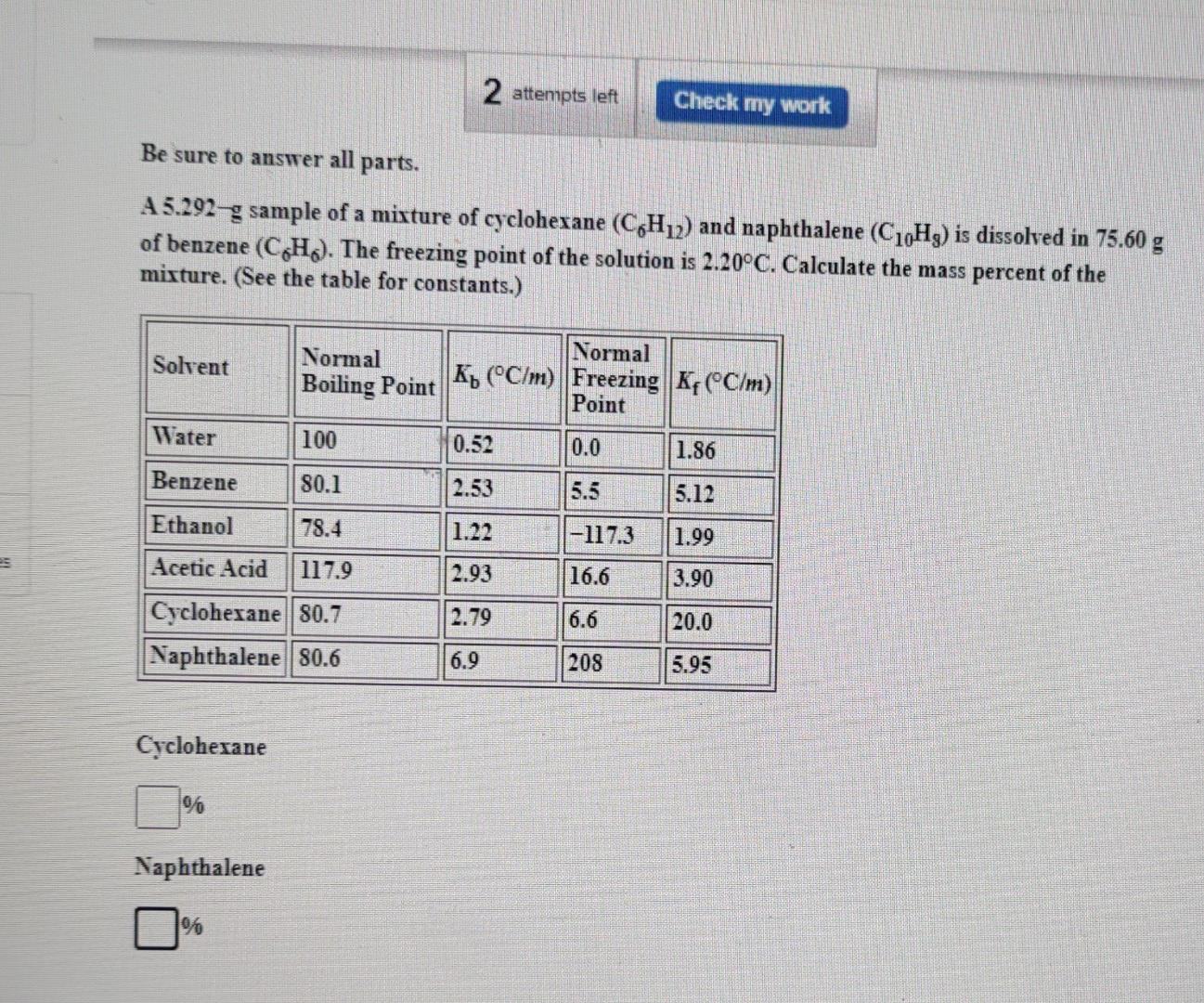
2 attempts left Check my work Be sure to answer all parts. A 5.292-g sample of a mixture of cyclohexane (C H12) and naphthalene (C10Hz) is dissolved in 75.60 g of benzene (C&H). The freezing point of the solution is 2.20C. Calculate the mass percent of the mixture. (See the table for constants.) Solvent Normal Normal Boiling Point Ko (C/m) Freezing K4 (C/m) Point Water 100 0.52 0.0 1.86 Benzene 80.1 2.53 5.5 5.12 Ethanol 78.4 1.22 -117.3 1.99 Acetic Acid 117.9 2.93 16.6 3.90 2.79 6.6 20.0 Cyclohexane 80.7 Naphthalene 80.6 6.9 208 5.95 Cycloherane % Naphthalene
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


