Question
2. Calculate (partition coefficient) for your mixture (use your data from part B): a. Use the deltaV and molarity of standardized HAc (aq) to calculate
2. Calculate (partition coefficient) for your mixture (use your data from part B): a. Use the deltaV and molarity of standardized HAc (aq) to calculate the moles of HAc in the aqueous phase before the extraction. Do this for each part B trial. b. Use the deltaV of NaOH (aq), the molarity of NaOH (aq) and the balanced reaction equation to calculate the moles of HAc in the aqueous phase after the extraction. Do this for each part B trial.
c. Subtract the step (b) value from the step (a) value. The result is the moles of HAc transferred to the TBME during the extraction. Do this for each part B trial. d. Calculate using information from the introduction and your data. Do this for each part B trial. e. Determine an average .
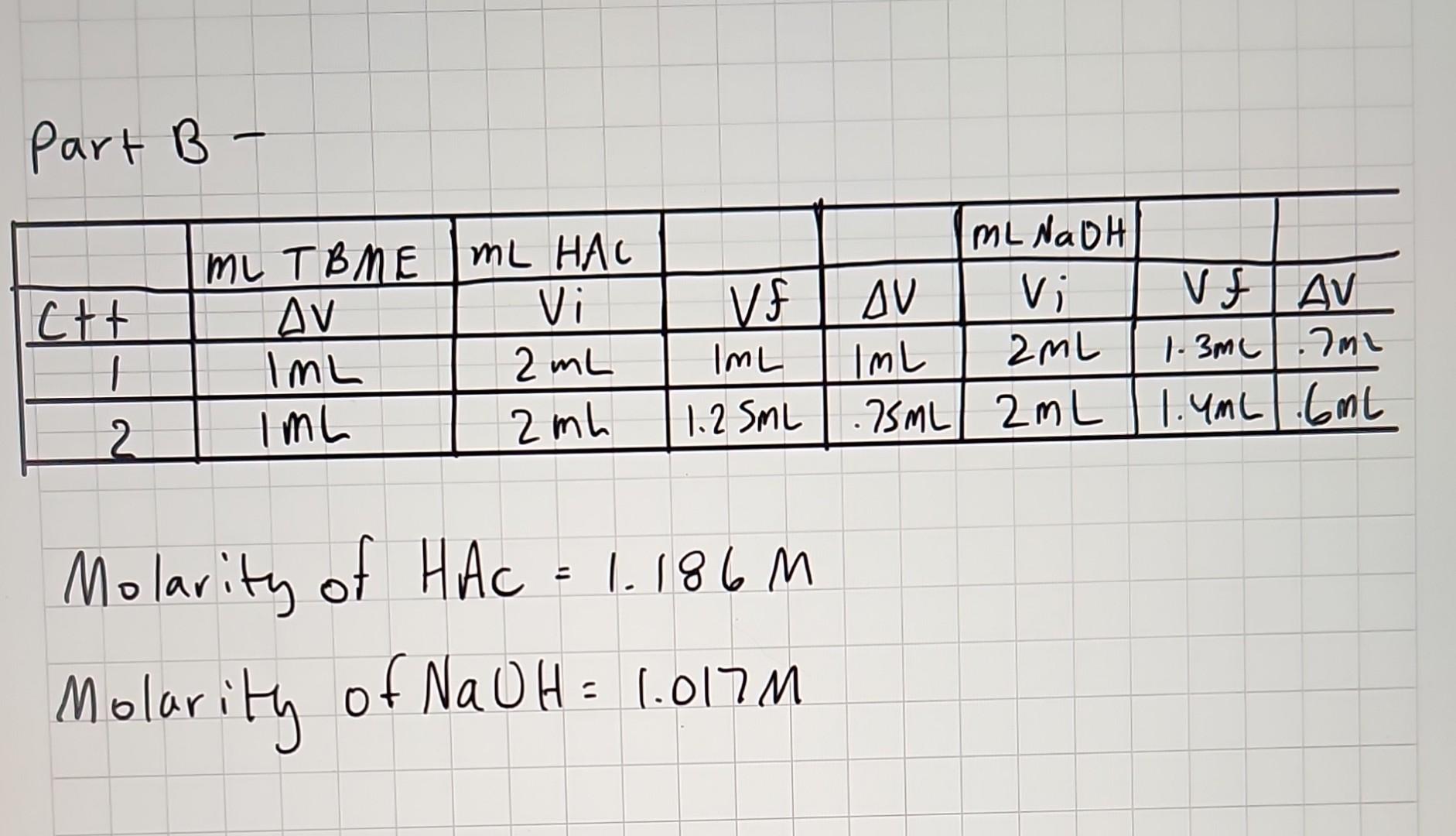
Part B - Molarity of HAC=1.186M Molarity of NaOH=1.017M
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


