Question
2. Consider the global NO formation reaction: N +0,2NO 2 d[NO] dt = k[N][O] Along with a thermal mechanism: (Zeldovich) (1) O+N NO+N E
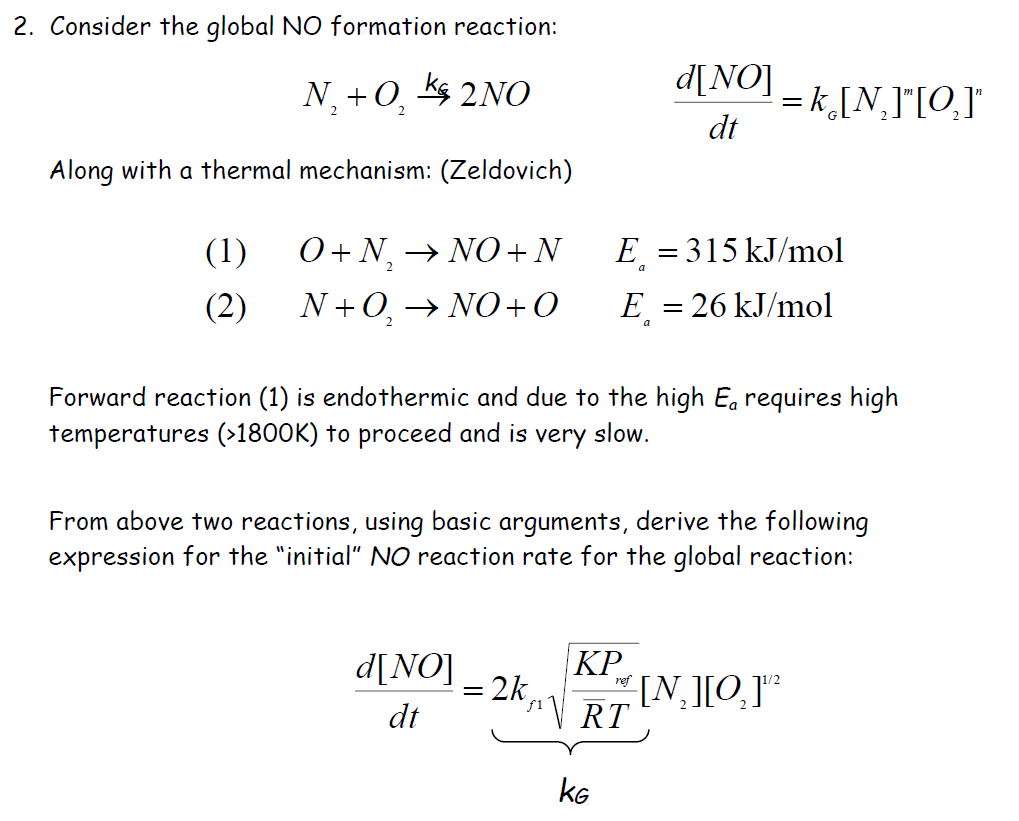
2. Consider the global NO formation reaction: N +0,2NO 2 d[NO] dt = k[N]"[O]" Along with a thermal mechanism: (Zeldovich) (1) O+N NO+N E = 315 kJ/mol (2) N+O NO+O E = 26 kJ/mol Forward reaction (1) is endothermic and due to the high E requires high temperatures (>1800K) to proceed and is very slow. From above two reactions, using basic arguments, derive the following expression for the "initial" NO reaction rate for the global reaction: d[NO] dt KP ref = = 2k [N][0]" fRT KG
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



