Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. Elaborate on the meaning of the empirical constants An and b in Moseley's law/rule for Ka radiation on the basis of a quasi-Bohr
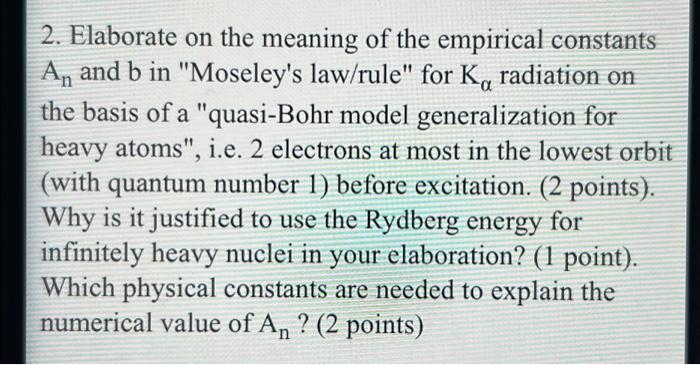
2. Elaborate on the meaning of the empirical constants An and b in "Moseley's law/rule" for Ka radiation on the basis of a "quasi-Bohr model generalization for heavy atoms", i.e. 2 electrons at most in the lowest orbit (with quantum number 1) before excitation. (2 points). Why is it justified to use the Rydberg energy for infinitely heavy nuclei in your elaboration? (1 point). Which physical constants are needed to explain the numerical value of An ? (2 points) 2. Elaborate on the meaning of the empirical constants An and b in "Moseley's law/rule" for Ko radiation on the basis of a "quasi-Bohr model generalization for heavy atoms", i.e. 2 electrons at most in the lowest orbit (with quantum number 1) before excitation. (2 points). Why is it justified to use the Rydberg energy for infinitely heavy nuclei in your elaboration? (1 point). Which physical constants are needed to explain the numerical value of An? (2 points)
Step by Step Solution
★★★★★
3.53 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Moseleys law formulated by Henry Moseley established a relationship between the wavelengths of characteristic Xray lines emitted by elements and their ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


