Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Flue gas (the gas that leaves a smoke stack after combustion) has a significant amount of COz that is typically released to the atmosphere.
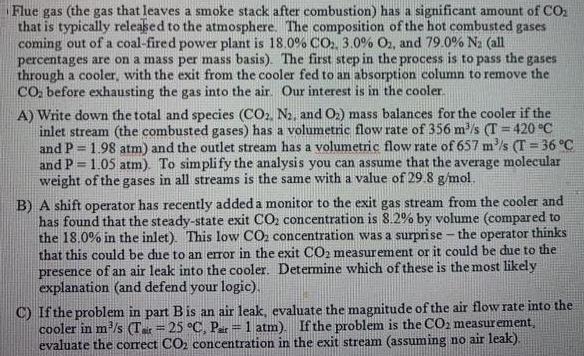
Flue gas (the gas that leaves a smoke stack after combustion) has a significant amount of COz that is typically released to the atmosphere. The composition of the hot combusted gases coming out of a coal-fired power plant is 18.0% CO, 3.0% O2, and 79.0% Na (all percentages are on a mass per mass basis). The first step in the process is to pass the gases through a cooler, with the exit from the cooler fed to an absorption column to remove the CO. before exhausting the gas into the air Our interest is in the cooler A) Write down the total and species (CO, N2, and O2) mass balances for the cooler if the inlet stream (the combusted gases) has a volumetric flow rate of 356 m/s (T = 420 C and P = 1.98 atm) and the outlet stream has a volumetric flow rate of 657 m/s (T = 36 C and P = 1.05 atm). To simplify the analysis you can assume that the average molecular weight of the gases in all streams is the same with a value of 29.8 g/mol. %3D %3D B) A shift operator has recently added a monitor to the exit gas stream from the cooler and has found that the steady-state exit CO, concentration is 8.2% by volume (compared to the 18.0% in the inlet). This low CO, concentration was a surprise - the operator thinks that this could be due to an error in the exit CO2 measurement or presence of an air leak into the cooler. Determine which of these is the most likely explanation (and defend your logic). could be due to the C) Ifthe problem in part Bis an air leak, evaluate the magnitude of the air flow rate into the cooler in m/s (Tar = 25 C, Par = 1 atm). If the problem is the CO2 measurement, evaluate the correct CO2 concentration in the exit stream (assuming no air leak). %3D %3D
Step by Step Solution
★★★★★
3.48 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
It should be noted that an Ideal gas Mass ratio and the molar r...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


