Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A coal power plant has a heat rate of 9700 Btu/kWh using coal which has an energy content of 24,000 kJ/kg. Burning coal results
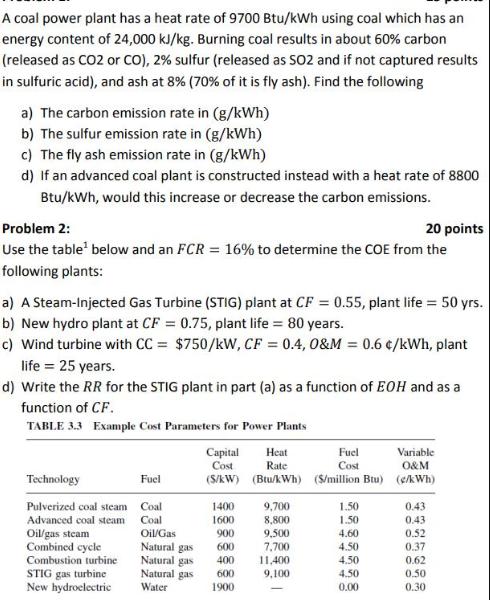
A coal power plant has a heat rate of 9700 Btu/kWh using coal which has an energy content of 24,000 kJ/kg. Burning coal results in about 60% carbon (released as CO2 or CO), 2% sulfur (released as SO2 and if not captured results in sulfuric acid), and ash at 8% (70% of it is fly ash). Find the following a) The carbon emission rate in (g/kWh) b) The sulfur emission rate in (g/kWh) c) The fly ash emission rate in (g/kWh) d) If an advanced coal plant is constructed instead with a heat rate of 8800 Btu/kWh, would this increase or decrease the carbon emissions. Problem 2: 20 points Use the table below and an FCR = 16% to determine the COE from the following plants: a) A Steam-Injected Gas Turbine (STIG) plant at CF = 0.55, plant life = 50 yrs. b) New hydro plant at CF = 0.75, plant life = 80 years. c) Wind turbine with CC = $750/kW, CF = 0.4, 0&M = 0.6 /kWh, plant life = 25 years. d) Write the RR for the STIG plant in part (a) as a function of EOH and as a function of CF. TABLE 3.3 Example Cost Parameters for Power Plants Technology Pulverized coal steam Advanced coal steam Oil/gas steam Combined cycle Combustion turbine STIG gas turbine New hydroelectric Fuel Coal Coal Oil/Gas Natural gas Heat Capital Cost Rate (S/kW) (Btu/kWh) ($/million Btu) (c/kWh) 1400 1600 900 600 Natural gas 400 Natural gas 600 Water 1900 9,700 8,800 9,500 7,700 11,400 9,100 Fuel Cost 1.50 1.50 4.60 4.50 4.50 4.50 0.00 Variable O&M 0.43 0.43 0.52 0.37 0.62 0.50 0.30
Step by Step Solution
★★★★★
3.45 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
Question 1a The carbon emission rate in gkWh is 714 To calculate the carbon emission rate we need to know the following The heat rate of the coal power plant 9700 BtukWh The energy content of coal 240...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


