Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. How much energy does it take to raise the temperature of one gram of water by 2.5C? Express your result in calories and
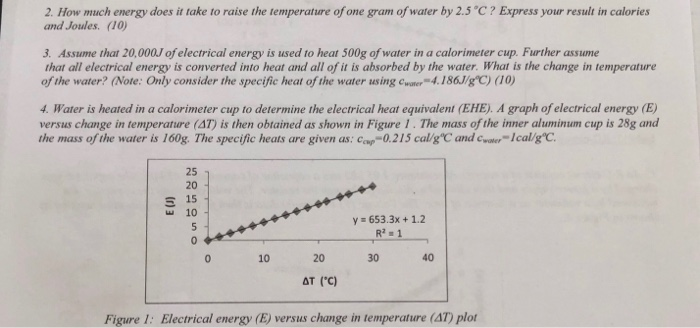
2. How much energy does it take to raise the temperature of one gram of water by 2.5C? Express your result in calories and Joules. (10) 3. Assume that 20,000J of electrical energy is used to heat 500g of water in a calorimeter cup. Further assume that all electrical energy is converted into heat and all of it is absorbed by the water. What is the change in temperature of the water? (Note: Only consider the specific heat of the water using water-4.186J/gC) (10) 4. Water is heated in a calorimeter cup to determine the electrical heat equivalent (EHE). A graph of electrical energy (E) versus change in temperature (AT) is then obtained as shown in Figure 1. The mass of the inner aluminum cup is 28g and the mass of the water is 160g. The specific heats are given as: Cap-0.215 cal/gC and water-Ical/gC. E 15 10 225050 y=653.3x+1.2 R2-1 0 10 20 30 AT (C) 40 Figure 1: Electrical energy (E) versus change in temperature (AT) plot
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


