Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. In order to study the photochemical decay of aqueous bromine in bright sunlight, a small quantity of liquid bromine was dissolved in water contained
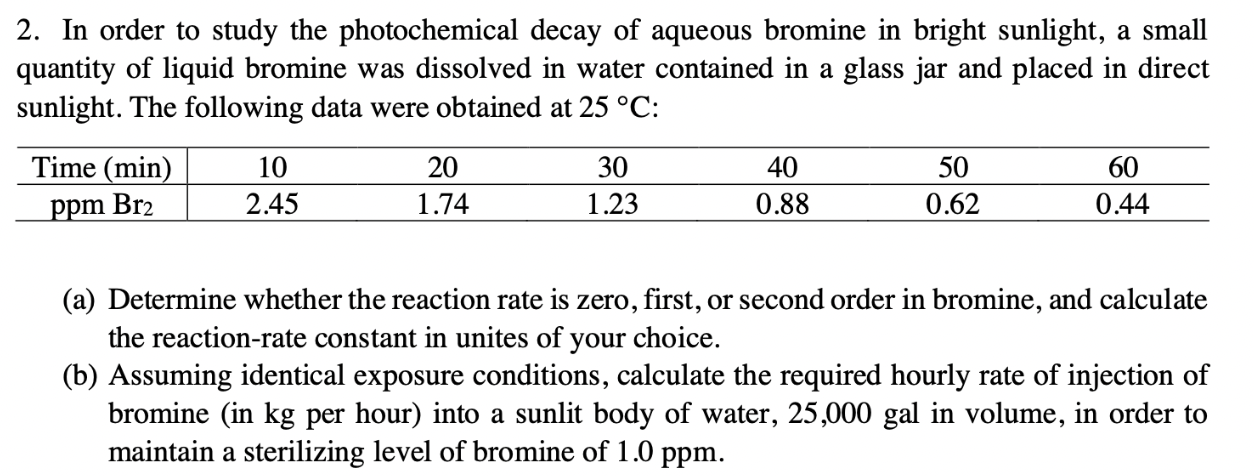 2. In order to study the photochemical decay of aqueous bromine in bright sunlight, a small quantity of liquid bromine was dissolved in water contained in a glass jar and placed in direct sunlight. The following data were obtained at 25C : T (a) Determine whether the reaction rate is zero, first, or second order in bromine, and calculate the reaction-rate constant in unites of your choice. (b) Assuming identical exposure conditions, calculate the required hourly rate of injection of bromine (in kg per hour) into a sunlit body of water, 25,000 gal in volume, in order to maintain a sterilizing level of bromine of 1.0ppm
2. In order to study the photochemical decay of aqueous bromine in bright sunlight, a small quantity of liquid bromine was dissolved in water contained in a glass jar and placed in direct sunlight. The following data were obtained at 25C : T (a) Determine whether the reaction rate is zero, first, or second order in bromine, and calculate the reaction-rate constant in unites of your choice. (b) Assuming identical exposure conditions, calculate the required hourly rate of injection of bromine (in kg per hour) into a sunlit body of water, 25,000 gal in volume, in order to maintain a sterilizing level of bromine of 1.0ppm Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


