Answered step by step
Verified Expert Solution
Question
1 Approved Answer
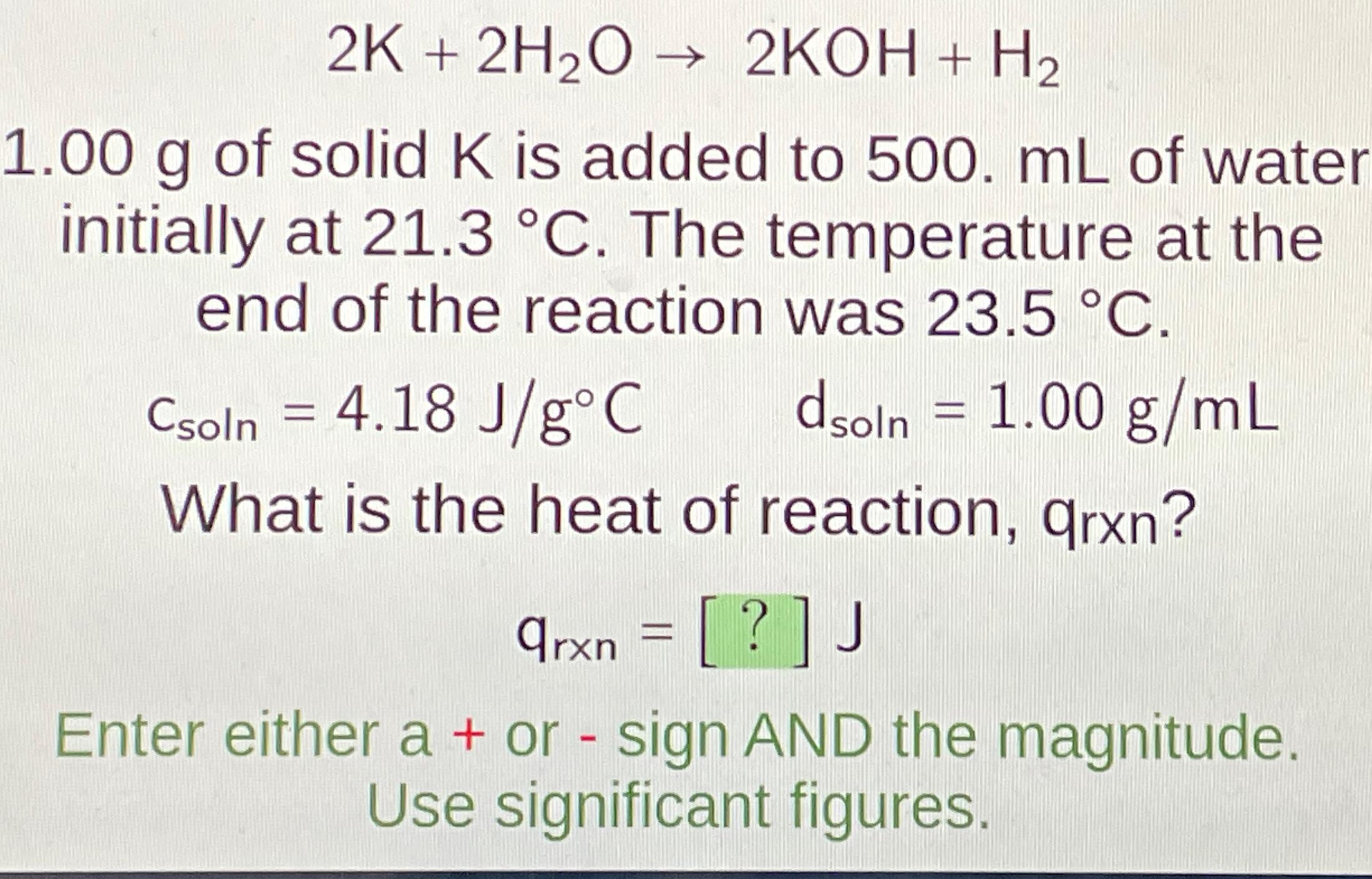
2 K + 2 H 2 O 2 KOH + H 2 1 . 0 0 g of solid K is added to 5 0
KOH
of solid is added to of water initially at The temperature at the end of the reaction was
What is the heat of reaction, qrxn
Enter either a or sign AND the
magnitude. Use significant figures.
I have been stuck on this question for a day. Please help me find the answer I need the best chemistry teacher I can find.
I've gone through a few answers that are incorrect such as: for every number listed here I've entered
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


