Question
2 kg of steam (gaseous H 2 O) at its boiling point and a 23 kg solid iron bar initially at a temperature of -300
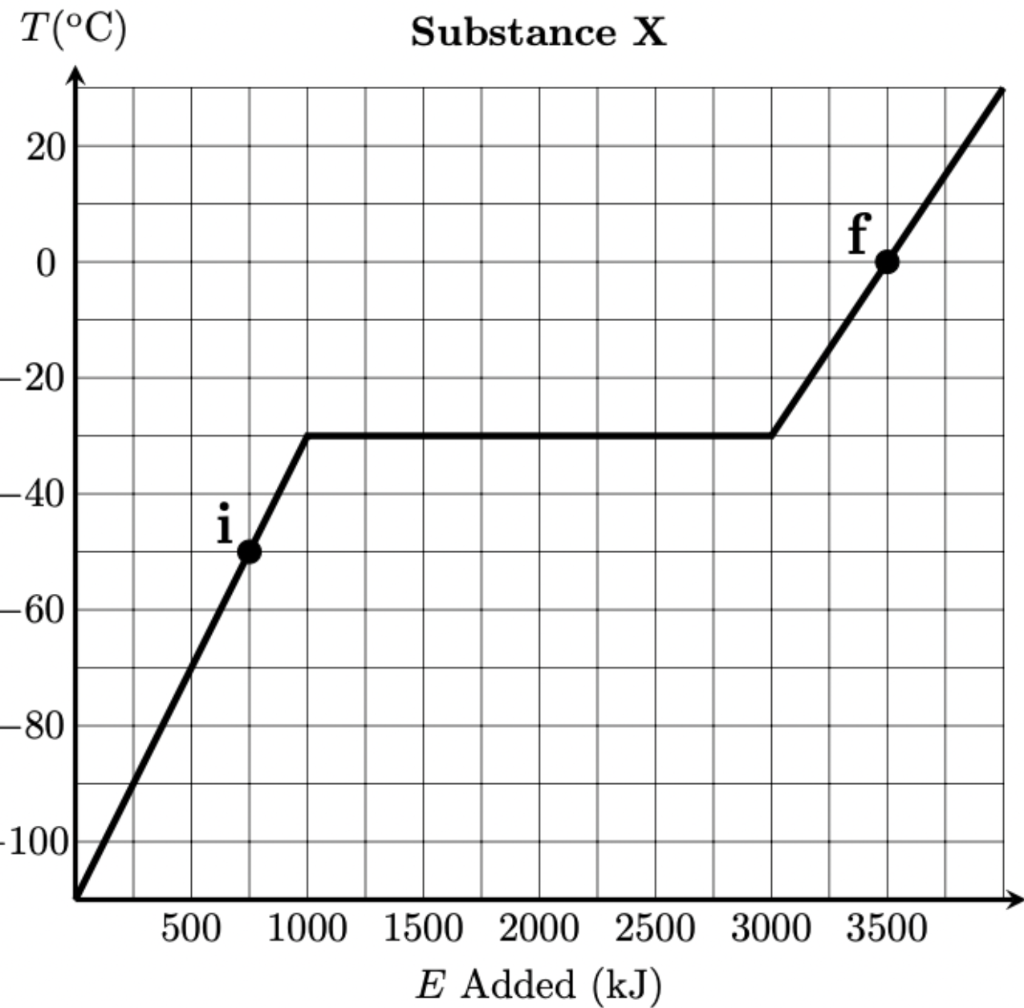
2 kg of steam (gaseous H2O) at its boiling point and a 23 kg solid iron bar initially at a temperature of -300oC are placed in a thermally insulated container with 6 kg of an unknown substance (called substance X) and allowed to reach thermal equilibrium. Substance X obeys the following Temperature Vs Energy Added diagram
The initial and final states of substance X are labeled (i and f) on the above diagram. Determine the final state (temperature and phase) of the H2O and the iron. If either substance ends in a mixed phase determine how much mass is in each phase.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


